- Oxycodone
-
Oxycodone 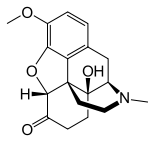

Systematic (IUPAC) name (5R,9R,13S,14S)-4,5α-epoxy-14-hydroxy-3-methoxy-17-methylmorphinan-6-one Clinical data Trade names Oxycodone AHFS/Drugs.com monograph MedlinePlus a682132 Pregnancy cat. B/D (prolonged use or in high doses at term) Legal status Controlled (S8) (AU) Schedule I (CA) ? (UK) Schedule II (US) Dependence liability Moderate–High Routes oral, intramuscular, intravenous, intranasal, subcutaneous, transdermal, rectal, epidural[1] Pharmacokinetic data Bioavailability 100% (IV); Up to 87% (oral)[2] Protein binding 45% Metabolism Hepatic: primarily CYP3A, secondarily CYP2D6[3] Half-life 3–4.5 hr Excretion Urine (19% unchanged) Identifiers CAS number 76-42-6 
ATC code N02AA05
N02AA55 (in combinations)PubChem CID 5284603 DrugBank DB00497 ChemSpider 4447649 
UNII CD35PMG570 
KEGG D05312 
ChEBI CHEBI:7852 
ChEMBL CHEMBL656 
Synonyms dihydrohydroxycodeinone, 14-hydroxydihydrocodeinone, 6-deoxy-7,8-dihydro-14-hydroxy-3-O-methyl-6-oxomorphine[4] Chemical data Formula C18H21NO4 Mol. mass 315.364 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Oxycodone is an opioid analgesic medication synthesized from opium-derived thebaine. It was developed in 1916 in Germany, as one of several new semi-synthetic opioids in an attempt to improve on the existing opioids: morphine, diacetylmorphine (heroin), and codeine.[1]
Oxycodone oral medications are generally prescribed for the relief of moderate to severe pain. Currently it is formulated as single ingredient products or compounded products. Some common examples of compounding are oxycodone with acetaminophen/paracetamol or NSAIDs such as ibuprofen. The formulations are available as generics but are also made under various brand names. OxyContin is Purdue Pharma's brand for time-release oral oxycodone. The manufacturing rights to time-released generic oxycodone are under dispute.
Contents
Medical uses
Oxycodone is effective for managing moderate to moderately severe acute or chronic pain.[5] It has been found to improve quality of life for those with many types of pain.[6]
In 2001, the European Association for Palliative Care recommended that oral oxycodone be a second-line alternative to oral morphine for cancer pain.[7] There is no evidence that any opioids are superior to morphine in relieving the pain of cancer, and no controlled trials have shown oxycodone to be superior to morphine.[8]
Adverse effects
The most commonly reported effects include memory loss, constipation, fatigue, dizziness, nausea, lightheadedness, headache, dry mouth, anxiety, pruritus, and diaphoresis.[10] It has also been claimed to cause dimness in vision due to miosis. Some patients have also experienced loss of appetite, nervousness, abdominal pain, diarrhea, ischuria, dyspnea, and hiccups,[2] although these symptoms appear in less than 5% of patients taking oxycodone. Rarely, the drug can cause impotence, enlarged prostate gland, and decreased testosterone secretion. Compared to morphine, oxycodone causes less respiratory depression, sedation, pruritus, and nausea.[11] As a result, it is generally better tolerated than morphine.[12]
In high doses, overdoses, or in patients not tolerant to opiates, oxycodone can cause shallow breathing, bradycardia, cold, clammy skin, apnea, hypotension, miosis (pupil constriction), circulatory collapse, respiratory arrest, and death.[2]
Withdrawal
There is a high risk of experiencing severe withdrawal symptoms if a patient discontinues oxycodone abruptly. Therefore therapy should be discontinued gradually rather than abruptly. People who use oxycodone in a hazardous or harmful fashion are at even higher risk of severe withdrawal symptoms as they tend to use higher than prescribed doses. The symptoms of oxycodone withdrawal are the same as for other opiate based painkillers and may include "anxiety, panic attack, nausea, insomnia, muscle pain, muscle weakness, fevers, and other flu like symptoms."[13]
Withdrawal symptoms have also been reported in newborns whose mothers had been either injecting or orally taking oxycodone during pregnancy.[14]
Detection in biological fluids
Oxycodone and/or its major metabolites may be quantitated in blood or urine to monitor for abuse, confirm a diagnosis of poisoning or assist in a medicolegal death investigation. Many commercial opiate screening tests cross-react appreciably with oxycodone and its metabolites, but chromatographic techniques can easily distinguish oxycodone from other opiates.[15]
Pharmacology
Mechanism of action
A group of Australian researchers has proposed (based on a 1997 study in rats) that oxycodone, unlike morphine (the effect of which is mediated by μ-opioid receptors), acts on κ-opioid receptors.[16] Further research by this group indicates the drug appears to be a κ2b-opioid agonist.[17] However, this has been disputed, primarily on the basis that oxycodone produces effects typical of μ-opioid agonists.[18]
Research by a Japanese group suggests that the effect of oxycodone is mediated by different receptors in different situations. Specifically, in diabetic mice the κ-opioid receptor appears to be involved in the antinociceptive effects of oxycodone,[19] while in non-diabetic mice the μ1-opioid receptor seems to be primarily responsible for these effects.[20]
Absorption
After a dose of conventional oral oxycodone, peak plasma levels of the drug are attained in approximately one hour;[3] in contrast, after a dose of OxyContin (an oral continuous release formulation), peak plasma levels of oxycodone occur in about three hours.[2]
Distribution
Oxycodone in the blood is distributed to skeletal muscle, liver, intestinal tract, lungs, spleen, and brain.[2] Conventional oral preparations of oxycodone start to reduce pain within 10–15 minutes; in contrast, OxyContin starts to reduce pain within 1 hour.[5]
Metabolism
Oxycodone is metabolized to α and β oxycodol; oxymorphone, then α and β oxymorphol and noroxymorphone; and noroxycodone, then α and β noroxycodol and noroxymorphone (N-desmethyloxycodone).[3] (14-Hydroxydihydrocodeine that in turn becomes 14-Hydroxydihydromorphine) These metabolites are true only for humans.[21] As many as six metabolites for oxycodone (14-hydroxydihydromorphinone, 14-hydroxydihydrocodeine, 14-hydroxydihydrocodeinone N-oxide {oxycodone N-oxide}, 14-hydroxydihydroisocodeine, 14-hydroxydihydrocodeine N-oxide, and noroxycodone) have been found in rabbits,[22] several of which are thought to be active metabolites to some extent, although a study using conventional oral oxycodone concluded that oxycodone itself, and not its metabolites, is predominantly responsible for the drug's opioid effects on the brain.[3]
Unlike morphine and hydromorphone, oxycodone is metabolized by the cytochrome P450 enzyme system in the liver, making it vulnerable to drug interactions.[2] Some people are fast metabolizers resulting in reduced analgesic effect but increased adverse effects, while others are slow metabolisers resulting in increased toxicity without improved analgesia.[23][24] The dose of OxyContin must be reduced in patients with reduced hepatic function.[5]
Elimination
Oxycodone and its metabolites are mainly excreted in the urine and sweat; therefore, it accumulates in patients with renal impairment.[5]
Dosage and administration
Oxycodone can be administered orally, intranasally, via intravenous/intramuscular/subcutaneous injection or rectally. The bioavailability of oral administration of Oxycodone averages 60–87%, with rectal administration yielding the same results; intranasal varies between individuals with a mean of 46%.[25]
Oxycodone is approximately 1.5–2 times as potent as morphine when administered orally.[26][27] However, 10–15 mg of oxycodone produces an analgesic effect similar to 10 mg of morphine when administered intramuscularly.[28] Therefore, as a parenteral dose, morphine is approximately up to 50% more potent than oxycodone.
There are no comparative trials showing that oxycodone is more effective than any other opioid. In palliative care, morphine remains the gold standard;[8] however, oxycodone can be useful as an alternative opioid if a patient has troublesome adverse effects with morphine.
Chemistry
Oxycodone's chemical name is derived from codeine. The chemical structures are very similar, differing only in that
- Oxycodone has a hydroxyl group at carbon-14 (codeine has just a hydrogen in its place), hence oxycodone;
- Oxycodone has a 7,8-dihydro feature, whereas codeine has a double bond between those two carbons; and
- Oxycodone has a carbonyl group (as in ketones) in place of the hydroxyl group of codeine, hence the "-one" suffix.
It is also similar to hydrocodone, differing only in that it has a hydroxyl group at carbon-14.[5]
Expanded expression for the compound oxycodone in the academic literature include "dihydrohydroxycodeinone",[4][29][30] "Eucodal",[29][30] "Eukodal",[1][31] "14-hydroxydihydrocodeinone",[4][29] and "Nucodan".[29][30] In a UNESCO convention, the translations of "oxycodone" are oxycodon (Dutch), oxycodone (French), oxicodona (Spanish), الأوكسيكودون (Arabic), 羟考酮 (Chinese), and оксикодон (Russian).[32] The word "oxycodone" should not be confused with "oxandrolone", "oxazepam", "oxybutynin", "oxytocin", or "Roxanol".[33]
History
Freund and Speyer of the University of Frankfurt in Germany first synthesized oxycodone from thebaine in 1916,[34] a few years after the German pharmaceutical company Bayer had stopped the mass production of heroin due to hazardous use, harmful use, and dependence. It was hoped that a thebaine-derived drug would retain the analgesic effects of morphine and heroin with less dependence. To some extent this was achieved, as oxycodone does not have the same immediate effect as heroin or morphine nor does it last as long.
The first clinical use of the drug was documented in 1917.[31] It was first introduced to the US market in May 1939. In early 1928, Merck introduced a combination product containing scopolamine, oxycodone, and ephedrine under the German initials for the ingredients SEE, which was later renamed Scophedal (SCOpolamine ePHEDrine & eukodAL)—this combination is essentially an oxycodone analogue of the morphine-based Twilight Sleep with ephedrine added to reduce circulatory and respiratory effects.
The International Narcotics Control Board estimates that 11.5 tons[clarification needed] of oxycodone were manufactured worldwide in 1998, which grew to 75.2 tons in 2007.[35] Of all countries, the United States had the highest total consumption of oxycodone in 2007 (82% of the world total of 51.6 tons).This translates into over half a billion 80mg tablets per year. [35] In addition, in 2007 the U.S. had the highest per capita consumption of oxycodone, followed by Canada, Denmark, Australia, and Norway.[35]
Society and culture
Brand names
All brand-names not marked otherwise are standard-release tablets or capsules of oxycodone without further pharmacologically active ingredients.
- Dinarkon (Germany)
- Diphydrone
- Endocet (US) (compounded with paracetamol)—by Endo
- Endodan (US) (compounded with acetylsalicylic acid)—by Endo
- ETH-Oxydose (US)
- Endone (US)—by Endo
- Eukodal (Germany) (for injection)
- OxyContin (US) (modified release)—by Purdue Frederick
- OxyFast (US) (oral liquid)
- OxyNorm (US) (tablets, oral liquid, liquid for injection)
- OxyNormoro (France)—by Mundipharma
- Pancodine
- Percodan (US) (compounded with acetylsalicylic acid)—by Endo
- Percodan-Demi (US) (compounded with acetylsalicylic acid)—by Endo
- Percocet (US) (compounded with paracetamol)—by Endo
- Percolone (US)—by Endo
- Roxicet (US)—by Roxane
- Roxicodone (US)—by Roxane
- Roxilox (US)—by Roxane
- Roxiprin (US) (compounded with acetylsalicylic acid)—by Roxane
- Supeudol (Canada)—by Sandoz
- Thekodin (Germany)
- Tylox (US)
OxyContin
OxyContin is the brand name of a time-release formula of oxycodone produced by the pharmaceutical company Purdue Pharma.[36] It was approved by the U.S. Food and Drug Administration in 1995 and first introduced to the U.S. market in 1996.[36] By 2001, OxyContin was the best-selling non-generic narcotic pain reliever in the U.S.; 2008 sales in the U.S. totaled $2.5 billion.[37] An analysis of data from the U.S. Drug Enforcement Administration found that retail sales of oxycodone "jumped nearly six-fold between 1997 and 2005."[38] Mundipharma distributes OxyContin in Australia,[39] China,[40] and Europe.[41]
In 2001, Purdue Pharma permanently suspended distribution of 160 mg tablets in the U.S. because of the "possibility of illicit use of tablets of such high strength." It is speculated that the DEA had requested Purdue to discontinue manufacturing the 160 mg Tablets, however the DEA has publicly denied asking Purdue to do so.[36][42] Beginning in 2010, the brand name OxyContin by Purdue was reformulated to prevent the misuse and abuse of the tablets. Additional binders have been added to prevent the grinding of tablets for insufflation or injection, and to maintain OxyContin's extended release characteristics.[43] These new tablets are more often prescribed by physicians than previous generic versions for this exact purpose and bear the stamp 'OP' instead of the previous 'OC.'
Lawsuits concerning generics
Purdue has multiple patents for OxyContin, but has been involved in a series of ongoing legal battles on the validity of these patents. On June 7, 2005, the United States Court of Appeals for the Federal Circuit upheld a decision from the previous year that some of Purdue’s patents for OxyContin could not be enforced.[44] This decision allowed and led to the immediate announcement from Endo Pharmaceuticals that would begin launching a generic version of all four strengths of OxyContin.[45] Purdue, however, had already made negotiations with another pharmaceutical company (IVAX Pharmaceuticals) to distribute their brand OxyContin in a generic form.[45] This contract was severed, and as of October 2005 Watson Pharmaceuticals became the exclusive U.S. distributor of Purdue-manufactured generic versions of OxyContin tablets in 10-, 20-, 40-, and 80-milligram dosages.[46]
On February 1, 2006, the Federal Circuit Court of Appeals issued a decision revising its 2005 decision.[47] This time the court vacated the lower court's "judgment that the patents-in-suit are unenforceable due to inequitable conduct," and the case was "remanded for further proceedings."[47]
Purdue Pharma has since announced resolution of its infringement suits with Endo,[48] Teva,[49] IMPAX,[50] and Mallinckrodt.[51] Endo and Teva each agreed to cease selling generic forms of OxyContin.[48][49] IMPAX negotiated a temporary, and potentially renewable, license.[50] In 2008, Mallinckrodt Pharmaceuticals reintroduced generic OxyContin in the strengths of 10 mg, 20 mg, 40 mg and 80 mg, which was made possible by a temporary royalties-bearing license with Purdue Pharma that expired in 2009.[51]
Marketing and misbranding
Critics have accused Purdue Pharma of putting profits ahead of public interest by applying "significant political pressure" to attempt to reverse South Carolina's requiring prior approval before a person with Medicaid can receive the drug;[52] for "fail[ing] to adequately warn consumers of the risks" of OxyContin such as dependence;[53] and for promoting the drug "aggressively" and by means such as "promotional beach hats, pedometers and swing-music CDs."[53][54]
In May 2007 Purdue Pharma "agreed to pay $19.5 million" in fines relating to aggressive off-label marketing practices of OxyContin in 26 states and the District of Columbia.[55] In specific, the company encouraged dosing more frequent than the recommended interval of 12 hours, and did not fully disclose the risk of hazardous or harmful use.[55]
Later in May 2007 Purdue Pharma and three of its top executives pleaded guilty in a Virginia federal court to charges that they misbranded OxyContin by representing it to have "less euphoric effect and less abuse potential" than it actually has, and by claiming that people taking the drug at low doses could stop taking it suddenly without symptoms of withdrawal.[56] The FDA had not approved these claims.[57] The company and the executives were to pay $634 million in fines for felony and misdemeanor misbranding.[56]
In October 2007, officials in Kentucky filed a lawsuit against Purdue Pharma for misleading health care providers and consumers "regarding the appropriate uses, risks and safety of OxyContin"; as of mid-2008, however, the case had been "consolidated with other lawsuits into a single multi-litigation suit" in a federal court in New York.[58]
Other preparations
Oxy·IR immediate-release oxycodone tablets from Purdue Pharma in Canada are available in 5, 10, and 20 mg strengths.[59] Effective August 10, 2009, Purdue discontinued manufacture and distribution of OxyIR Capsules.
OxyNorm is available in 5, 10, and 20 mg capsules, and also as a 5 mg/5 ml liquid in 250 ml bottles in Australia, New Zealand, the Netherlands and the UK.[60][61][62] In addition, OxyNorm is available in a 10 mg/ml liquid concentrate for oral use in the Netherlands and the UK, and in 10 mg/ml solutions and 50 mg/ml solutions for injection or infusion in New Zealand, the Netherlands and the UK.[62][63] It is also available in Australia as Endone, a generic 5 mg tablet.
Percocet (oxycodone with paracetamol/acetaminophen) tablets are available in Canada and the U.S. with 2.5, 5, 7.5, and 10 mg of oxycodone and varying amounts of acetaminophen.[64]
Depalgos (oxycodone with paracetamol) tablets are marketed in Italy, with 325 mg Paracetamol and 5, 10, and 20 mg oxycodone. Recent legislation in Italy has made it easier for physicians to prescribe this medication and other opioids to pain patients.[65]
Percodan tablets available in the U.S. contain 4.8355 mg of oxycodone HCl and 325 mg of aspirin.[66]
Proladone suppositories, available in Australia, contain 30 mg of oxycodone pectinate.[67]
Roxicodone, a generic oxycodone product designed to have an immediate release effect. The "R" in roxycodone stands for rapid, which is the only difference.It is available in 5 mg (white), 15 mg (green), and 30 mg (blue) tablets; in a 5 mg per 5 ml oral solution; and in a 20 mg per ml liquid concentrate.[68][69] On March 31, 2009, the U.S. Food and Drug Administration directed Boehringer Ingelheim Roxane and Xanodyne Pharmaceuticals to cease manufacture and distribution of 5 mg Roxicodone tablets in the U.S. because they lacked proper approval.[70] In the US Oxycodone Immediate Release tablets are also available in 10 mg (pink) and 20 mg (grey) tablets from KVK-Tech, a generic manufacturer. Targin is a tablet with a prolonged-release oxycodone/naloxone combination.
Recreational use
- General Info
Although the effects, addiction, and chemical composition of oxycodone are extremely similar to heroin, oxycodone misuse often lacks the strong taboos and negative reputation of heroin.[citation needed] Heroin's reputation has been developed over years of observing the detrimental effects on heroin abusers' lives.[citation needed] Lacking the reputation of heroin, many youths and novice drug users engage in oxycodone abuse without understanding the consequences associated with its abuse,[citation needed] such as a heroin-like addiction potential and the threat of a fatal overdose. The appeal to youth and novice drug users is principally due to its ease of use (oral, intranasal) and its availability.[citation needed] Also, pharmaceutical drugs like oxycodone have a definite purity and known ingredients, unlike street-drugs like heroin, which have relatively unknown purity and ingredients. There are many different varieties of the 30MG Roxy. Such manufacturers as Mallincrockt, Amide, and Qualitest among others produce these pills.
- Australia
The illegal use of OxyContin began in Australia in the early 2000s. By 2007, 51% of a national sample of injection drug users in Australia had reported using oxycodone, and 27% had injected it in the last six months.[71]
File:Qualitest Pharmaceuticals OxycodoneHCL 30mg.jpgGeneric Oxycodone 30MG frequently abused by addicts.- Canada
Deaths from opioid pain relievers increased from 13.7 deaths per million residents in 1991 to 27.2 deaths per million residents in 2004, which may be related to the spread of OxyContin.[72]
- United Kingdom
Hazardous use, harmful use and diversion of OxyContin in the UK commenced in the early- to mid-2000s.[73] The first known death due to OxyContin overdose in the UK occurred in 2002.[74]
- United States
Instances of recreational use and diversion of OxyContin have increased in the U.S. beginning in the late 1990s.[75] The slang term hillbilly heroin (which originally referred to hydromorphone) for OxyContin refers to the occurrence of the "earliest reported cases of Oxycontin abuse" in the U.S. in rural areas such as Appalachia.[76] Diversion of OxyContin in the U.S. may occur through "fraudulent prescriptions, doctor shopping, over-prescribing, and pharmacy theft."[75]
A 2003 study by the Government Accountability Office found three factors that may have contributed to the illicit use and distribution of OxyContin in the U.S.:[36]
- OxyContin contains a large amount of oxycodone compared with other types of oxycodone containing pills.
- OxyContin's warning label said to not crush the controlled-release tablets because of the potential for rapid release of oxycodone, which led to many people crushing the tablets and injecting or snorting the drug.
- By 2001, sales of OxyContin in the U.S. exceeded $1 billion per year.
- Oxycontin remains within your system from seven to ten days after consumed.
A study published in 2005 examined the prevalence of opiate analgesic use among "recreational drug users and street addicts" as perceived by "key informants" throughout the U.S.; the authors found that non-clinical use of opiates was increasing in general, but that of the drugs studied use of OxyContin "was mentioned most frequently."[77] Purdue Pharma has attempted to reformulate the 10–40 mg strengths of OxyContin to prevent the release of a high percentage of the oxycodone by crushing; however, in 2008 a joint panel convened by the U.S. Food and Drug Administration was "concerned that abusers could find a way to manipulate the new formulation."[78] In 2010, Purdue Pharma introduced 'Oxycontin OP', Purdue Pharma predicts that abuse of higher dose formulations such as 'OxyContin' will go down, but this change may cause heavy recreational users to resort to other drugs such as heroin or may increase the number of deaths caused by acetaminophen poisoning from users attempting to take similar doses of drugs like 'Percocet' or 'Vicodin' to get similar effects. Since 'Oxycontin OP' has now fully saturated the street markets, recreational users find it nearly impossible to find the original Oxycontin formulations.
One investigation in Boston found that OxyContin was a "gateway" drug for heroin, which addicts turned to as a cheaper alternative.[79]
Legal status
Oxycodone is subject to international conventions on narcotic drugs. In addition, oxycodone is subject to national laws that differ by country. The 1931 Convention for Limiting the Manufacture and Regulating the Distribution of Narcotic Drugs of the League of Nations included oxycodone.[80] The 1961 Single Convention on Narcotic Drugs of the United Nations, which replaced the 1931 convention, categorized oxycodone in Schedule I.[81] Global restrictions on Schedule I drugs include "limit[ing] exclusively to medical and scientific purposes the production, manufacture, export, import, distribution of, trade in, use and possession of" these drugs; "requir[ing] medical prescriptions for the supply or dispensation of [these] drugs to individuals"; and "prevent[ing] the accumulation" of quantities of these drugs "in excess of those required for the normal conduct of business."[81]
- Australia
Oxycodone is in Schedule I (derived from the Single Convention on Narcotic Drugs) of the Commonwealth's Narcotic Drugs Act 1967.[82] In addition, it is in Schedule 8 of the Australian Standard for the Uniform Scheduling of Drugs and Poisons ("Poisons Standard"), meaning that it is a "controlled drug... which should be available for use but require[s] restriction of manufacture, supply, distribution, possession and use to reduce abuse, misuse and physical or psychological dependence."[83]
- Canada
Oxycodone is a controlled substance under Schedule I of the Controlled Drugs and Substances Act (CDSA).[84] Every person who seeks or obtains from a practitioner either the substance or an authorization to obtain the substance must disclose to that practitioner information on all controlled substances and authorizations for controlled substances obtained from any other practitioner within the preceding 30 days; otherwise, the person may be found "guilty of an indictable offence and liable to imprisonment for a term not exceeding seven years".[84] Anyone possessing the substance for the purpose of trafficking "is guilty of an indictable offence and liable to imprisonment for life".[84]
- Germany
The drug is in Appendix III of the Narcotics Act ("Betäubungsmittelgesetz" or BtMG).[85] The law states that only physicians, dentists and veterinarians ("Ärzte, Zahnärzte und Tierärzte") can prescribe oxycodone, and that the federal government can regulate the prescriptions (e.g., by requiring reporting).[85]
- Hong Kong
Oxycodone is regulated under Part I of Schedule 1 of Hong Kong's Chapter 134 Dangerous Drugs Ordinance.[86] The penalty for trafficking (Section 4) or manufacturing (Section 6) the substance is a $5,000,000 HKD fine and/or life imprisonment.[86] In Section 8 of the Ordinance, possession of the substance for consumption without licence from the Department of Health is illegal and subject to a $1,000,000 HKD fine and/or 7 years of imprisonment.[86] Per Sections 22–23, only specific health professionals and others (e.g., "a person in charge of a laboratory used for the purposes of research") may possess and supply the substance.[86] Anyone who supplies the substance without a valid prescription can be fined $10,000 HKD according to Section 31.[86]
- Singapore
Oxycodone is listed as a Class A drug in the Misuse of Drugs Act of Singapore, which means that offences in relation to the drug attract the most severe level of punishment. A conviction for unauthorized manufacture of the drug attracts a minimum sentence of ten years' imprisonment and corporal punishment of five strokes of the cane, and a maximum sentence of life imprisonment or 30 years' imprisonment and 15 strokes of the cane.[87] The minimum and maximum penalties for unauthorized trafficking in the drug are respectively five years' imprisonment and five strokes of the cane, and 20 years' imprisonment and 15 strokes of the cane.[88]
- United Kingdom
Oxycodone is a Class A drug under the Misuse of Drugs Act.[89] For Class A drugs, which are "considered to be the most likely to cause harm," possession without a prescription is punishable by up to seven years in prison, an unlimited fine, or both.[90] Dealing of the drug illegally is punishable by up to life imprisonment, an unlimited fine, or both.[90] In addition, oxycodone is a Schedule 2 drug per the Misuse of Drugs Regulations 2001 which "provide certain exemptions from the provisions of the Misuse of Drugs Act 1971."[91]
- United States
Under the Controlled Substances Act, oxycodone is a Schedule II drug because it "has a high potential for abuse," because it "has a currently accepted medical use in treatment in the United States or a currently accepted medical use with severe restrictions," and because use of the drug "may lead to severe psychological or physical dependence."[92] According to Section 829 of the Act, Schedule II drugs must be dispensed only with the written prescription of a practitioner except in certain situations (e.g., "dispensed directly by a practitioner, other than a pharmacist," or "dispensed upon oral prescription (i.e. telephone)" in "emergency situations only").[92] Furthermore, Section 829 specifies that prescriptions for Schedule II drugs cannot be refilled.[92]
As of April 2010 an "abuse resistant", controlled release formulation of Oxycontin was approved for sale in the United States containing Butylated hydroxytoluene, or BHT. The reformulated OxyContin is intended to lessen the ability to tamper with the opioid medication — from being cut, broken, chewed, crushed or dissolved, however, there is no evidence that the reformulation of OxyContin is less subject to misuse, abuse, diversion, overdose, or addiction.[93] The abuse resistant formulation is recognizable by a pill marking "OP" on one side and the milligrams of oxycodone contained within the pill on the other. The previous formulation has an "OC" marking replacing the "OP" of the new formulation.[94] BHT is listed as a "known carcinogen" in Canada and Mexico, and a 'possible carcinogen' in the United States.[95] Only the United States and Japan use the BHT 'OP' formulation, likely due to cancer and other health concerns.
See also
Notes
This article uses the terms "hazardous use," "harmful use," and "dependence" in accordance with Lexicon of alcohol and drug terms published by the World Health Organization (WHO) in 1994.[96] In WHO usage, the first two terms replace the term "abuse" and the third term replaces the term "addiction."[96]References
- ^ a b c Kalso, E (2005). "Oxycodone". Journal of Pain and Symptom Management 29 (5S): S47–S56. doi:10.1016/j.jpainsymman.2005.01.010. PMID 15907646.
- ^ a b c d e f (PDF) 1. Package insert Oxycontin. Stamford, CT: Purdue Pharma L.P. 2007-11-05. http://www.purduepharma.com/PI/Prescription/Oxycontin.pdf. Retrieved 2009-03-23.
- ^ a b c d Lalovic B, Kharasch E, Hoffer C, Risler L, Liu-Chen LY, Shen DD (2006). "Pharmacokinetics and pharmacodynamics of oral oxycodone in healthy human subjects: role of circulating active metabolites" (PDF). Clin Pharmacol Ther 79 (5): 461–479. doi:10.1016/j.clpt.2006.01.009. PMID 16678548. http://paincenter.wustl.edu/c/BasicResearch/documents/KharashClinPharm06.pdf. Retrieved 2009-03-28.
- ^ a b c Maryadele J. O'Neil, editor ... (2006). The Merck index (14 ed.). Whitehouse Station, NJ: Merck & Co.. ISBN 978-0-911910-00-1.
- ^ a b c d e "Oxycodone". The American Society of Health-System Pharmacists. http://www.drugs.com/monograph/oxycodone.html. Retrieved 3 April 2011.
- ^ Riley, J; Eisenberg, E; Müller-Schwefe, G; Drewes, AM; Arendt-Nielsen, L (2008). "Oxycodone: a review of its use in the management of pain". Curr Med Res Opin 24 (1): 175–192. doi:10.1185/030079908X253708. PMID 18039433.
- ^ Hanks, GW; Conno, F; Cherny, N; Hanna, M; Kalso, E; Mcquay, HJ; Mercadante, S; Meynadier, J et al. (2001-03-02). "Morphine and alternative opioids in cancer pain: the EAPC recommendations: Expert Working Group of the Research Network of the European Association for Palliative Care" (PDF). Br J Cancer 84 (5): 587–593. doi:10.1054/bjoc.2001.1680. PMC 2363790. PMID 11237376. http://www.nature.com/bjc/journal/v84/n5/pdf/6691680a.pdf.
- ^ a b Derek Doyle, etc., (Ed.). (1999). Calman K. Doyle D, Hanks G, Editors. ed. Oxford Textbook of Palliative Medicine. Oxford: Oxford University Press. ISBN 0192625667.
- ^ American Society of Health-System Pharmacists (2009-03-23). "Oxycodone". U.S. National Library of Medicine, MedlinePlus. http://www.nlm.nih.gov/medlineplus/druginfo/meds/a682132.html. Retrieved 2009-03-27.
- ^ Oxycodone Side Effects.
- ^ "Oxycodone Professional Monograph – FDA". Drugs.com. http://www.drugs.com/pro/oxycodone.html. Retrieved 2010-08-01.
- ^ "Commonsense Oxycodone Rx & Safety" (PDF). http://pain-topics.org/pdf/OxycodoneRxSafety.pdf. Retrieved 2010-08-01.
- ^ "Oxycodone". Center for Substance Abuse Research. 2005-05-02. http://www.cesar.umd.edu/cesar/drugs/oxycodone.asp. Retrieved 2009-03-25.
- ^ Rao R, Desai NS (June 2002). "OxyContin and neonatal abstinence syndrome" (PDF). J Perinatol 22 (4): 324–5. doi:10.1038/sj.jp.7210744. PMID 12032797. http://www.nature.com/jp/journal/v22/n4/pdf/7210744a.pdf. Retrieved 2009-03-25.
- ^ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 9th edition, Biomedical Publications, Foster City, CA, 2011, pp. 1259–1262.
- ^ Ross FB, Smith MT (1997). "The intrinsic antinociceptive effects of oxycodone appear to be κ-opioid receptor mediated". Pain 73 (2): 151–157. doi:10.1016/S0304-3959(97)00093-6. PMID 9415500.
- ^ Smith MT (2008). "Differences between and combinations of opioids re-visited". Curr Opin Anaesthesiol 21 (5): 596–601. doi:10.1097/ACO.0b013e32830a4c4a. PMID 18784485.
- ^ Kalso E (2007). "How different is oxycodone from morphine?". Pain 132 (3): 227–228. doi:10.1016/j.pain.2007.09.027. PMID 17961923.
- ^ Nozaki C, Saitoh A, Kamei J (2006). "Characterization of the antinociceptive effects of oxycodone in diabetic mice". Eur. J. Pharmacol. 535 (1–3): 145–151. doi:10.1016/j.ejphar.2006.02.002. PMID 16533506.
- ^ Nozaki C, Kamei J (2007). "Involvement of mu1-opioid receptor on oxycodone-induced antinociception in diabetic mice". Eur. J. Pharmacol. 560 (2–3): 160–162. doi:10.1016/j.ejphar.2007.01.021. PMID 17292346.
- ^ Moore, KA; Ramcharitar, V; Levine, B; Fowler, D (2003). "Tentative identification of novel oxycodone metabolites in human urine". Journal of analytical toxicology 27 (6): 346–52. PMID 14516487.
- ^ T Ishida, K Oguri and H Yoshimura (1979). "Isolation and identification of urinary metabolites of oxycodone in rabbits". Drug Metab. Dispos. 7 (3): 162–165. PMID 38087. http://dmd.aspetjournals.org/cgi/content/abstract/7/3/162.
- ^ Gasche Y, Daali Y, Fathi M, et al. (2004). "Codeine intoxication associated with ultrarapid CYP2D6 metabolism". N Engl J Med 351 (27): 2827–31. doi:10.1056/NEJMoa041888. PMID 15625333.
- ^ Otton SV, Wu D, Joffe RT, Cheung SW, Sellers EM (1993). "Inhibition by fluoxetine of cytochrome P450 2D6 activity". Clin Pharmacol Ther 53 (4): 401–9. doi:10.1038/clpt.1993.43. PMID 8477556.
- ^ Analgesic Expert Group. Therapeutic Guidelines: Analgesic. Version 4. Melbourne: Therapeutic Guidelines Ltd, 2007.
- ^ (OXYCODONE HCl CONTROLLED-RELEASE) TABLETS
- ^ Palliative Care Perspectives. James L. Hallenbeck.
- ^ "Oxycodone Hydrochloride Tablets USP 5 mg, 15 mg, & 30 mg" (PDF). Mallinckrodt Inc. 2007-08-10. http://pharmaceuticals.mallinckrodt.com/_attachments/PackageInserts/57_Oxy%20HCl%20Tabs_REV081007.pdf. Retrieved 2009-03-24.
- ^ a b c d Eddy NB (1973). The National Research Council involvement in the opiate problem, 1928–1971. Washington: National Academy of Sciences.
- ^ a b c May EL, Jacobson AE (1989). "The Committee on Problems of Drug Dependence: a legacy of the National Academy of Sciences. A historical account". Drug Alcohol Depend 23 (3): 183–218. doi:10.1016/0376-8716(89)90083-5. PMID 2666074.
- ^ a b Sunshine A, Olson NZ, Colon A, Rivera J, Kaiko RF, Fitzmartin RD, Reder RF, Goldenheim PD, A; Olson, NZ; Colon, A; Rivera, J; Kaiko, RF; Fitzmartin, RD; Reder, RF; Goldenheim, PD (1 July 1996). "Analgesic efficacy of controlled-release oxycodone in postoperative pain". J Clin Pharmacol 36 (7): 595–603. PMID 8844441. http://jcp.sagepub.com/cgi/reprint/36/7/595. Retrieved 2009-03-25.
- ^ United Nations Educational, Scientific and Cultural Organization (2005). "International convention against doping in sport" (PDF). http://unesdoc.unesco.org/images/0014/001425/142594m.pdf. Retrieved 2009-04-04.
- ^ Hicks RW, Becker SC, Cousins DD, eds. (2008) (PDF). MEDMARX data report. A report on the relationship of drug names and medication errors in response to the Institute of Medicine’s call for action. Rockville, MD: Center for the Advancement of Patient Safety, US Pharmacopeia. http://www.usp.org/pdf/EN/medmarx/2008MEDMARXReport.pdf. Retrieved 2009-04-04.
- ^ Sneader W (2005). Drug discovery: a history. Hoboken, NJ: Wiley. p. 119. ISBN 0471899801.
- ^ a b c International Narcotics Control Board (2009) (PDF). Narcotic drugs: estimated world requirements for 2009; statistics for 2007. Report E/INCB/2008/2. New York: United Nations. ISBN 978-92-1-048124-3. http://www.incb.org/pdf/technical-reports/narcotic-drugs/2008/narcotics_drugs_2008.pdf.
- ^ a b c d (PDF) Prescription drugs. OxyContin abuse and diversion and efforts to address the problem. Report GAO-04-0110. Washington, DC: U.S. Government Accounting Office. December 2003. http://www.gao.gov/new.items/d04110.pdf. Retrieved 2008-03-28.
- ^ "Details for Oxycontin". Drugpatentwatch.com. http://drugpatentwatch.com/ultimate/preview/tradename/index.php?query=OXYCONTIN. Retrieved 2010-08-01.
- ^ Bass F, Associated Press (2007-08-20). "AP: pain medicine use has nearly doubled". Washington Post. http://www.washingtonpost.com/wp-dyn/content/article/2007/08/20/AR2007082000147.html. Retrieved 2009-04-16.
- ^ Nader, Carol (2007-03-02). "Drug users target powerful painkiller". The Age (Melbourne, Australia). http://www.theage.com.au/news/national/drug-users-target-powerful-painkiller/2007/03/01/1172338796110.html. Retrieved 2009-04-09.
- ^ Yu SY, OxyContin Tablets Postmarketing Surveillance Study Group China (2008). "Postmarketing surveillance study of OxyContin tablets for relieving moderate to severe cancer pain". Oncology 74 (Suppl 1): 46–51. doi:10.1159/000143218. PMID 18758197.
- ^ Mundipharma International Ltd (2006-11-03). "Mundipharma receives boost to European OxyContin(R) patent position: Teva withdraws challenge from European patent office". PR Newswire. http://www.prnewswire.co.uk/cgi/news/release?id=183302. Retrieved 2009-04-09.
- ^ Reidy, Maurice Timothy (2001-05-11). "Maker acts on controversial painkiller tablets – 160 mg tablets of Oxycontin drew DEA attention". Hartford Courant.
- ^ "FDA Approves New Formulation for OxyContin". fda.gov. 2010-04-05. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm207480.htm. Retrieved 2011-01-14.
- ^ Purdue Pharma L.P. v. Endo Pharms. Inc, 410 F.3d 690 (Fed. Cir. 2005-06-07).
- ^ a b "Purdue comments on Federal Court of Appeal decision on OxyContin patent litigation" (Press release). Purdue Pharma. 2005-06-08. http://www.pharma.com/pressroom/news/20050608.htm. Retrieved 2009-04-11.
- ^ "Purdue appoints Watson Pharmaceuticals exclusive distributor of authorized generic versions of OxyContin tablets" (Press release). Purdue Pharma. 2005-10-28. http://www.pharma.com/pressroom/news/20051028.htm. Retrieved 2009-04-11.
- ^ a b Purdue Pharma L.P. v. Endo Pharms. Inc., 438 F.3d 1123 (Fed. Cir. 2006-02-01).
- ^ a b "Purdue Pharma L.P. announces resolution of OxyContin patent lawsuit with Endo Pharmaceuticals" (Press release). Purdue Pharma. 2006-08-28. http://www.purduepharma.com/pressroom/news/20060828-1.htm. Retrieved 2009-04-11.
- ^ a b "Purdue Pharma L.P. announces signing of consent judgment ending OxyContin tablets patent lawsuit with Teva Pharmaceuticals" (Press release). Purdue Pharma. 2006-10-16. http://www.purduepharma.com/pressroom/news/20061016.htm. Retrieved 2009-04-11.
- ^ a b "Purdue Pharma L.P. announces agreement to end OxyContin patent lawsuit with IMPAX laboratories" (Press release). Purdue Pharma. 2007-04-02. http://www.purduepharma.com/pressroom/news/20070402.htm. Retrieved 2009-04-11.
- ^ a b "Purdue Pharma L.P. announces resolution of OxyContin patent lawsuit with Mallinckrodt Inc." (Press release). Purdue Pharma. 2009-04-11. http://www.purduepharma.com/pressroom/news/20080902.htm. Retrieved 2009-04-11.
- ^ Bauerlein V (2001-09-23). "Popular painkiller mired in controversy" (reprint). The State. http://www.mapinc.org/drugnews/v01/n1702/a06.html. Retrieved 2009-03-29.
- ^ a b Rosenberg D (2001-07-02). "Drugs: profits vs. pain relief. Does its maker push Oxycontin too hard?". Newsweek. http://www.newsweek.com/id/78596. Retrieved 2009-04-11.
- ^ "Editorial: selling drugs legally, but not always safely" (reprint). Roanoke Times. 2001-06-13. http://www.mapinc.org/drugnews/v01/n1052/a08.html. Retrieved 2009-04-11.
- ^ a b "Drugmaker to pay $19.5 mil to settle OxyContin lawsuit". Arizona Republic. Associated Press. 2007-05-09. http://www.azcentral.com/arizonarepublic/business/articles/0509biz-oxycontin0509.html. Retrieved 2009-04-11.
- ^ a b O'Brien J (2007-05-10). "Purdue pleads out, will pay $634 million in fines". LegalNewsline.com. http://www.legalnewsline.com/news/194919-purdue-pleads-guilty-will-pay-634-million-in-fines. Retrieved 2009-04-11.
- ^ Chasan E (2007-05-10). "Purdue Frederick pleads guilty in OxyContin case". Reuters. http://www.reuters.com/article/healthNews/idUSWBT00695020070510. Retrieved 2009-04-21.
- ^ Moore C (2008-06-11). "OxyContin lawsuit stuck in N.Y. court". Appalachian News-Express. http://www.news-expressky.com/articles/2008/06/11/news/01court.txt. Retrieved 2009-04-21.
- ^ (PDF) Product monograph [OxyContin and OxyIR]. Pickering, Ontario: Purdue Pharma. 2008-08-20. http://www.purdue.ca/pdf/2008-08-20%20OxyContin%20and%20OxyIR%20PM_FINAL_ENG.pdf. Retrieved 2009-03-23.
- ^ "Search results [for OxyNorm in Pharmaceutical Benefits Schedule"]. Department of Health and Ageing, Australian Government. 2008-06-16. http://www.pbs.gov.au/html/industry/search/results?term=OxyNorm. Retrieved 2009-03-31.
- ^ "OXYNORM". New Zealand Medicines and Medical Devices Safety Authority. 2010-05-21. http://www.medsafe.govt.nz/profs/datasheet/o/oxynormcapsoln.pdf. Retrieved 2011-07-23.
- ^ a b "Search results [for OxyNorm in electronic Medicines Compendium."]. Datapharm Communications Ltd. http://emc.medicines.org.uk/searchresults.aspx?term=oxynorm&searchtype=QuickSearch. Retrieved 2009-03-31.
- ^ "OXYNORM injection". New Zealand Medicines and Medical Devices Safety Authority. 2010-04-13. http://www.medsafe.govt.nz/profs/datasheet/o/oxynorminj.pdf. Retrieved 2011-07-23.
- ^ "Percocet (oxycodone and acetaminophen tablets, USP)" (PDF). Endo Pharmaceuticals. 2006. http://www.endo.com/pdf/products/Percocet_pack_insert_2.pdf. Retrieved 2009-03-24.
- ^ "Ordinanza 16 Giugno 2009" (PDF). Ministero Della Salute. 2009. http://www.sirfarma.it/binary/sirfarma/normativa/Ordinanza_Stupefacenti_Sottosegretario_Fazio_16.06.2009.pdf.
- ^ "Percodan (oxycodone and aspirin tablets, USP)" (PDF). Endo Pharmaceuticals. 2005. http://www.endo.com/pdf/products/percodan_pack_insert.pdf. Retrieved 2009-03-25.
- ^ Phebra Pty Ltd (2009-10-29). "Product information Proladone" (PDF). http://www.phebra.com.au/data/products/TAB007-pi.pdf. Retrieved 2011-07-23.
- ^ "Roxicodone" (PDF). Xanodyne Pharmaceuticals Inc. February 2006. http://www.xanodyne.com/pdf/Roxicodone-5mg20mg-orals-tablet.pdf. Retrieved 2009-03-31.
- ^ "Roxicodone" (PDF). Roxane Laboratories, Inc. http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/021011s002lbl.pdf. Retrieved 2011-07-23.
- ^ "Questions and answers for consumers about FDA’s action involving unapproved narcotics containing morphine sulfate, hydromorphone, or oxycodone". U.S. Food and Drug Administration. 2009-03-31. http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/EnforcementActivitiesbyFDA/SelectedEnforcementActionsonUnapprovedDrugs/ucm165587.htm. Retrieved 2011-07-23.
- ^ Black E, et al. (2008) (PDF). Australian drug trends 2007. Findings from the Illicit Drug Reporting System (IDRS). Sydney: National Drug and Alcohol Research Centre, University of New South Wales. ISBN 9780733426254. http://ndarc.med.unsw.edu.au/NDARCWeb.nsf/resources/DRUG_TRENDS_1_NAT/$file/DT001.PDF. Retrieved 2009-04-06.
- ^ "Study finds huge rise in oxycodone deaths". CTV News. http://www.ctv.ca/servlet/ArticleNews/story/CTVNews/20091207/opioids_091207/20091207?hub=TopStoriesV2. Retrieved 2009-12-07.
- ^ Gordon T (2008-03-30). "Scots' use of 'hillbilly heroin' rises by 430%". Sunday Times (London).
- ^ Thompson T (2002-03-24). "Epidemic fear as 'hillbilly heroin' hits the streets". Society Guardian. http://www.guardian.co.uk/society/2002/mar/24/drugsandalcohol. Retrieved 2009-04-16.
- ^ a b "Action plan to prevent the diversion and abuse of OxyContin". U.S. Drug Enforcement Administration. 2001-06-22. http://www.deadiversion.usdoj.gov/drugs_concern/oxycodone/abuse_oxy.htm. Retrieved 2009-03-31.
- ^ Tough P (2001-07-29). "The alchemy of OxyContin". The New York Times. http://www.nytimes.com/2001/07/29/magazine/the-alchemy-of-oxycontin.html. Retrieved 2009-04-16.
- ^ Cicero TJ, Inciardi JA, Muñoz A (2005). "Trends in abuse of OxyContin and other opioid analgesics in the United States: 2002–2004" (PDF). J Pain 6 (10): 662–72. doi:10.1016/j.jpain.2005.05.004. PMID 16202959. http://paincenter.wustl.edu/c/BasicResearch/documents/CiceroJPain2005.pdf.
- ^ Smith L (2008-06-01). "Panel slams OxyContin trials and says abuse, mortality risks persist". Family Practice News 38 (11): 5. doi:10.1016/S0300-7073(08)70694-7.
- ^ "OxyContin A Gateway For Young Users In Eastie". WBUR. http://www.wbur.org/2010/04/12/east-boston-oxycontin. Retrieved 2010-08-01.
- ^ League of Nations (1931). "Convention for limiting the manufacture and regulating the distribution of narcotic drugs" (PDF). http://treaties.un.org/doc/Treaties/1931/07/19310713%2006-44%20AM/Ch_VI_8_ap.pdf. Retrieved 2009-04-04.
- ^ a b "United Nations conference for the adoption of a single convention on narcotic drugs. Final act" (PDF). 1961. http://treaties.un.org/doc/Treaties/1964/12/19641213%2002-14%20AM/Ch_VI_15p.pdf. Retrieved 2009-04-04.
- ^ Commonwealth of Australia. "Narcotic Drugs Act 1967 – first schedule". Australasian Legal Information Institute. http://www.austlii.edu.au/au/legis/cth/consol_act/nda1967160/sch1.html. Retrieved 2009-04-06.
- ^ Australian Government. Department of Health and Aging. Therapeutic Goods Administration (June 2008) (PDF). Standard for the uniform scheduling of drugs and poisons no. 23. Canberra: Commonwealth of Australia. ISBN 1741865964. http://www.comlaw.gov.au/ComLaw/Legislation/LegislativeInstrument1.nsf/0/3BBB39C4645284BCCA2574A6001C711F/$file/PoisonsStandard2008.pdf. Retrieved 2009-04-06.
- ^ a b c Canada Department of Justice (2009-02-27). "Controlled Drugs and Substances Act (1996, c. 19)". http://laws.justice.gc.ca/en/ShowFullDoc/cs/C-38.8///en. Retrieved 2009-03-23.
- ^ a b German Federal Ministry of Justice (2009-01-19). "Act on the circulation of narcotics (Narcotics Act – BtMG)" (in German). http://bundesrecht.juris.de/btmg_1981/BJNR106810981.html. Retrieved 2009-04-06.
- ^ a b c d e Hong Kong Special Administrative Region, People's Republic of China. "Dangerous drugs ordinance – chapter 134". Hong Kong Legal Information Institute. http://www.hklii.org/hk/legis/en/ord/cur/134.txt. Retrieved 2009-04-08.
- ^ Misuse of Drugs Act (Cap. 185, 2008 Rev. Ed.) (Singapore), section 6(1).
- ^ Misuse of Drugs Act (Singapore), section 5(1).
- ^ "List of drugs currently controlled under the Misuse of Drugs legislation". UK. Home Office. 2009. http://www.homeoffice.gov.uk/documents/cdlist.pdf?view=Binary. Retrieved 2009-04-08.
- ^ a b "Class A, B and C drugs". UK. Home Office. http://www.homeoffice.gov.uk/drugs/drugs-law/Class-a-b-c/. Retrieved 2009-04-08.
- ^ "Statutory instrument 2001 No. 3998. The Misuse of Drugs regulations 2001". UK. Office of Public Sector Information. http://www.opsi.gov.uk/si/si2001/20013998.htm. Retrieved 2009-04-08.
- ^ a b c "(United States Code.) Title 21 – food and drugs. Chapter 13 – drug abuse prevention and control". U.S. Drug Enforcement Administration. http://uscode.house.gov/download/pls/21C13.txt. Retrieved 2011-07-23.
- ^ Letter from Purdue Pharma L.P. to Healthcare Professional, October 4, 2010
- ^ ""FDA Approves New Formulation of OxyContin Designed to reduce abuse of the prescription painkiller, though it has failed to stop the addiction epidemic. People are still finding ways to abuse the substance, or just turns to heroin to keep themselves from going into withdrawals.", consumeraffairs.com (April 6, 2010)". Consumeraffairs.com. 2010-04-06. http://www.consumeraffairs.com/news04/2010/04/oxycontin_fda.html. Retrieved 2010-08-01.
- ^ Butylated hydroxytoluene MSDS
- ^ a b Babor T, et al., compilers (1994) (PDF). Lexicon of alcohol and drug terms. Geneva: World Health Organization. ISBN 9241544686. http://whqlibdoc.who.int/publications/9241544686.pdf.
Further reading
- Meier, Barry (2003). Pain killer: a "wonder" drug's trail of addiction and death. Emmaus, PA: Rodale. ISBN 1579546382.
- Pinsky, Drew (2004). When painkillers become dangerous: what everyone needs to know about OxyContin and other prescription drugs. Center City, MN: Hazelden. ISBN 159285107X.
- Lockwood, Brad (2006). Oxycontin: from pain relief to addiction. New York: Rosen Pub. Group, Inc. ISBN 9781404209138.
External links
- Coluzzi F, Mattia C. Oxycodone. Pharmacological profile and clinical data in chronic pain management. Minerva Anestesiol 2005 Jul–Aug;71(7–8):451-60.
- Watch Cottonland, a National Film Board of Canada documentary on OxyContin addiction
Recreational drug use Major recreational drugs Amphetamine · Arecoline (Areca) · Betel · Caffeine (Coffee · Tea) · Cathinone (Khat) · Cocaine (Coca) · Ephedrine (Ephedra) · Mephedrone · Methamphetamine · Methylphenidate · Nicotine (Tobacco) · Theobromine (Cocoa)EntactogensHallucinogensBufotenin ( Psychoactive toads · Vilca · Yopo) · DMT (Ayahuasca) · LSA · LSD-25 · Mescaline (Peruvian Torch · Peyote · San Pedro) · Psilocybin / Psilocin (Psilocybin mushrooms)DXM · Inhalants (Nitrous oxide · alkyl nitrites – poppers, such as amyl nitrite) · Ketamine · Methoxetamine · Muscimol (Amanita muscaria) · PCP · Salvinorin A (Salvia divinorum)Atropine and Scopolamine (Datura · Deadly Nightshade · Henbane · Mandrake) · Dimenhydrinate · DiphenhydramineCannabinoids
Drug subculture 420 · Cannabis cultivation · Cannabis smoking · Legal history of cannabis in the United States · Legality of cannabis · Marijuana Policy Project · Medical cannabis · NORML · Religious and spiritual use of cannabis · Stoner filmOtherProblems with drug use Abuse · Dependence (Prevention · Opioid replacement therapy · Rehabilitation · Responsible use) · Drug-related crime · Fetal alcohol spectrum disorder · Illegal drug trade · Long-term effects of cannabis · Neurotoxicity · OverdoseLegality of drug use InternationalState levelDrug policy
by countryAustralia · Canada · Germany · Netherlands · Portugal · Sweden · Switzerland · Soviet Union · United States (Just Say No · Office of National Drug Control Policy · School district drug policies · California · Colorado · Maryland · Virginia)OtherLists of countries by... Alcohol consumption · Cannabis legality (Annual use · Lifetime use) · Cigarette consumption · Cocaine use · Opiates use
- Related navpages:
-
- Opioids (420 topics)
Categories:- Semisynthetic opioids
- Morphinans
- German inventions
- Euphoriants
- Ketones
- Alcohols
- Phenol ethers
- Mu-opioid agonists
Wikimedia Foundation. 2010.



