- Moxifloxacin
-
Moxifloxacin 
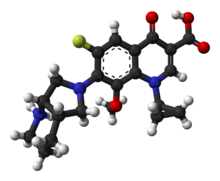
Systematic (IUPAC) name 1-cyclopropyl-7-[(1S,6S)-2,8-diazabicyclo [4.3.0]non-8-yl]-6-fluoro-8-methoxy-4-oxo- quinoline-3-carboxylic acid
Clinical data Pregnancy cat. C (United States)
B3 (Australia)Legal status Prescription Only Routes Oral, IV, local (eyedrops) Pharmacokinetic data Bioavailability 86 to 92% Protein binding 30 to 50% Metabolism Glucuronide and sulfate conjugation
Cytochrome P450 system not involvedHalf-life 12 hours Excretion hepatic Identifiers CAS number 354812-41-2 
ATC code J01MA14 S01AX22 PubChem CID 152946 DrugBank APRD00281 ChemSpider 134802 
UNII U188XYD42P 
ChEMBL CHEMBL32 
Chemical data Formula C21H24FN3O4 Mol. mass 401.431 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Moxifloxacin is a fourth-generation synthetic fluoroquinolone antibacterial agent developed by Bayer AG (initially called BAY 12-8039). It is marketed worldwide (as the hydrochloride) under the brand names Avelox, Avalox, and Avelon for oral treatment. In most countries, the drug is also available in parenteral form for intravenous infusion. Moxifloxacin is also sold in an ophthalmic solution (eye drops) under the brand names Vigamox, Moxeza for the treatment of conjunctivitis (pink eye).
A United States patent application was submitted on June 30, 1989 for Avelox (moxifloxacin hydrochloride),[1] but it was not until 1999 (ten years later) that Avelox was approved by the U.S. Food and Drug Administration (FDA) for use in the United States to treat severe and life threatening bacterial infections.[2]
The licensed uses for moxifloxacin are quite limited as moxifloxacin is to be considered a drug of last resort when all other antibiotics have failed.[3] There are currently only six approved uses in the adult population (three of which are restricted[4]) and Vigamox (ophthalmic) is the only one used for children. Moxifloxacin interacts with a number of other drugs, as well as a number of herbal and natural supplements.[5]
Avelox (moxifloxacin) was launched in the United States in 1999 and is currently marketed in more than 80 countries worldwide. In the United States, Avelox is marketed by Bayer's partner Merck. Within the past year (2008–2009) Bayer has issued three Dear Doctor Letters concerning severe adverse reactions associated with the use of moxifloxacin, as well as giving notice regarding restrictions in its use.[4][6][7] First marketed in 1999, moxifloxacin is Bayer's heir apparent to ciprofloxacin.
As of 2011 the FDA has added two boxed warnings for this drug in reference to spontaneous tendon ruptures and the fact that moxifloxacin may cause worsening of myasthenia gravis symptoms, including muscle weakness and breathing problems. Such an adverse reaction is a potentially life-threatening event and may require ventilatory support.[8]
Medical uses
Moxifloxacin is used to treat a number of infections including: respiratory tract infections, cellulitis, anthrax, intraabdominal infections, endocarditis, meningitis, and tuberculosis.[9]
In the adult population its oral and intravenous is limited to the treatment of proven serious and life-threatening bacterial infections. The initial approval by the FDA (December 1999)[10] encompassed the following indications:
Additional indications were approved by the FDA as follows:
- April, 2001: Uncomplicated Skin and Skin Structure Infections (uSSSI)[13]
- May, 2004: Community Acquired Pneumonia caused by multi-drug resistant Streptococcus pneumoniae.[14]
- June, 2005: Complicated Skin and Skin Structure Infections (cSSSI)[15]
- November, 2005: Complicated Intra-Abdominal Infections (cIAI).[16]
The licensed uses noted as restricted use requires that moxifloxacin only be prescribed when other antibiotics that have been initially recommended for treatment cannot be used or have failed.[3][4][17]
At the current time, there are no approved uses within the pediatric population for Oral and I.V. moxifloxacin. A significant number of drugs found within this class, including moxifloxacin, are not licensed by the FDA for use in children due to the risk of fatalities.[18][19] as well as permanent injury to the musculoskeletal system.[20]
Prescribing moxifloxacin to treat an unapproved use (other than those listed above) within the pediatric, as well as the adult population, does however take place rather frequently.
In ophthalmology, moxifloxacin licensed use is limited to the treatment of conjunctival infections caused by susceptible bacteria.[21]
Note: Moxifloxacin may be licensed for other uses, or restricted, by the various regulatory agencies worldwide.
Availability
Moxifloxacin is available as tablet, intravenous solution, and eye drop.
Adverse effects
See also: Adverse effects of fluoroquinolonesThe serious adverse effects that may occur as a result of moxifloxacin therapy include irreversible peripheral neuropathy, spontaneous tendon rupture and tendonitis,[22] acute liver failure or serious liver injury, QTc prolongation/torsades de pointes, toxic epidermal necrolysis (TEN), and clostridium difficile-associated disease (CDAD),[23] as well as photosensitivity/phototoxicity reactions.[24] Hepatitis, pseudomembranous colitis, psychotic reactions and Stevens-Johnson syndrome have also been associated with moxifloxacin therapy.[25]
There has been a number of regulatory actions taken as a result of such adverse reactions, which include published warnings,[26] the issuance of numerous "Dear Doctor Letters",[4][6][7][27] the restrictions regarding the use of moxifloxacin instituted by the European agency's Committee for Medicinal Products for Human Use (CHMP),[28] as well as the recent addition of Black Box Warnings.[29] On March 22, 2010 Health Canada issued a notice to health care professionals and Canadians regarding the recent changes to the labeling information for Avelox (moxifloxacin). The 2010 Canadian updated labeling now includes information regarding the risk of severe liver injury during moxifloxacin therapy.[30]
These serious events may occur with therapeutic or with acute overdose. Such adverse reactions may manifest during, as well as after moxifloxacin therapy.[31]
Most recently, the German regulatory authorities placed additional restrictions on the use of oral moxifloxacin in patients with acute bacterial sinusitis (ABS), acute exacerbation of chronic bronchitis (AECB), and community-acquired pneumonia (CAP) stating that in case of these diseases moxifloxacin should only be prescribed when other antibiotics that have been initially recommended for treatment cannot be used or have failed. Additional notice was given that rhabdomyolysis, the exacerbation of symptoms of myasthenia gravis and the risk of cardiac arrhythmia in women and older patients, was associated with moxifloxacin.[7] Currently the German regulatory authorities are investigating the association of severe and life threatening QTc prolongation/torsades de pointes with moxifloxacin therapy, which the FDA had raised serious concerns about during the initial drug approval process back in 1999.[32][33][34]
Serious visual complications have also been reported to occur with ophthalmic fluoroquinolone therapy, which may also occur with Vigamox, especially corneal perforation, but also evisceration and enucleation. Corneal perforation occurred most commonly in elderly patients with deep ulcers. These increased incidents of corneal perforation may be due to fluoroquinolones' causing alterations in stromal collagen, leading to a reduction in tectonic strength.[35][36]
Contraindications
The fluoroquinolone class is now considered to be contraindicated for the treatment of certain sexually transmitted diseases by some experts due to bacterial resistance.[37] Due to growing prevalence of antibiotic resistance to the fluoroquinolones in southeast Asia, the use of the drugs found within the fluoroquinolone class (which would include moxifloxacin) in patients having been to southeast Asia is increasingly being contraindicated.[37][38]
There are only two listed contraindications found within the 2008 package insert:
- "Nonsteroidal anti-inflammatory drugs (NSAIDs): Although not observed with moxifloxacin in preclinical and clinical trials, the concomitant administration of a nonsteroidal anti-inflammatory drug with a fluoroquinolone may increase the risks of CNS stimulation and convulsions." [39]
- "Moxifloxacin is contraindicated in persons with a history of hypersensitivity to moxifloxacin, any member of the quinolone class of antimicrobial agents, or any of the product components."[39]
Though not stated as such within the package insert, ziprasidone is also considered to be contraindicated, as it may have the potential to prolong QT interval. Moxifloxacin should also be avoided in patients with uncorrected hypokalemia, or concurrent administration of other medications known to prolong the QT interval (antipsychotics and tricyclic antidepressants).[40]
Moxifloxacin should be used with caution in patients suffering from diabetes, as glucose regulation may be significantly altered.[40]
Moxifloxacin is also considered to be contraindicated within the pediatric population, pregnancy, nursing mothers, patients with a history of tendon disorder, patients with documented QT prolongation,[7] and patients with epilepsy or other seizure disorders. Coadministration of moxifloxacin with other drugs that also prolong the QT interval or induce bradycardia (e.g., beta-blockers, amiodarone) should be avoided. Careful consideration should be given in the use of moxifloxacin in patients with cardiovascular disease, including those with conduction abnormalities.[40]
Recently (2008), Bayer issued a Europrean Dear Doctor Letter concerning moxifloxacin-associated liver damage, and, as such, the use of moxifloxacin would now be considered contraindicated in patients with impaired liver function.[4]
- Pregnancy
The fluoroquinolones rapidly cross the blood-placenta and blood-milk barrier, and are extensively distributed into the fetal tissues. For this reason, the fluroquinolones are contraindicated during pregnancy due to the risk of spontaneous abortions and birth defects. The flouroquinolones have also been reported as being present in the mother's milk and are passed on to the nursing child, which may increases the risk of the child's suffering from this syndrome as well, even though the child had never been prescribed or taken any of the drugs found within this class.[41][42]
- Pediatric population
Fluoroquinolones are not licensed by the FDA for use in children due to the risk of fatalities[19] as well as permanent injury to the musculoskeletal system, with two exceptions. Ciprofloxacin is being licensed for the treatment of Complicated Urinary Tract Infections and Pyelonephritis due to Escherichia coli and Inhalational Anthrax (post-exposure), and Levofloxacin was recently licensed for the treatment of Inhalational Anthrax (post-exposure). However, the fluoroquinolones are licensed to treat lower respiratory infections in children with cystic fibrosis in the UK. It does not appear that there have been any pediatric trials involving oral or IV moxifloxacin to date. But the fluoroquinolones that have been involved in pediatric clinical trials reveal the following:
Within one study it was stated that the pediatric patient has a 3.8% chance of experiencing a serious musculoskeletal adverse event.[43] Within the studies submitted in response to a Pediatric Written Request (Ciprofloxacin, circa 2004) the rate of athropy was reported to be 9.3%.[44]
Two recent pediatric studies involving the use of levofloxacin indicates that the pediatric patient has a greater than 50% chance of experiencing one or more adverse reactions. This would be consistent with the studies found within the NDA (new drug application) for Levofloxacin,[45] which showed and ADR rate in excess of 40%, as well as a number of reported fatalities. Within the first study,[18] it is stated that "Of the 712 subjects evaluable for safety, 275 (52%) levofloxacin-treated subjects experienced one or more adverse event... Serious adverse events were reported in 33 (6%) levofloxacin-treated subjects... Two serious adverse events in levofloxacin-treated subjects resulted in fatal outcomes." Within the second study,[46] it is stated that "Of the 204 subjects evaluable for safety, 122 experienced one or more adverse events.... Twelve subjects (6%) discontinued study drug due to an adverse event.... Seven subjects (3%) experienced 8 serious adverse events'." (circa 2007)
Within the BPCA Pediatric Studies Summary for ciprofloxacin,[44] it was stated that the overall incidence of adverse events at six weeks was 41%. This would be consistent with the safety profile found with the other fluoroquinolones studied in the pediatric population, as noted above with levofloxacin. As such, the current ban on the use of the fluoroquinolones in the pediatric population is both reasonable and supported by various clinical studies. The risk of permanent injury outweighs the potential benefits.
Interactions
Antacids containing aluminium or magnesium ions inhibit the absorption of moxifloxacin. Drugs that prolong the QT interval (e.g., pimozide) may have an additive effect on QT prolongation and lead to increased risk of ventricular arrhythmias. The INR (International Normalised Ratio) may be increased or decreased in patients treated with warfarin. Moxifloxacin has been shown in a number of case reports to interact with warfarin.[47] The exact mechanism for the warfarin-quinolone drug interaction is unknown.[48] A precautionary measure would be to monitor the INR more closely and, if necessary, adjust the anticoagulant dose as necessary. Moxifloxacin does not appear to inhibit theophylline metabolism.[49] However, caution may be warranted when using theophylline with all of the fluoroquinolones, including moxifloxacin. Drug Interaction Facts notes that some fluoroquinolones, especially ciprofloxacin, enoxacin, and norfloxacin, interact with theophylline.[48]
Significant interactions
Moxifloxacin is not believed to be associated with clinically significant drug interactions due to inhibition or stimulation of hepatic metabolism. Thus, it should not, for the most part, require special clinical or laboratory monitoring to ensure its safety.[50] However, there is an additive effect of moxifloxacin and drugs that prolong the QT interval, such as cisapride, erythromycin, antipsychotics, and tricyclic antidepressants. Therefore, moxifloxacin should be used with caution when given concurrently with such drugs. Moxifloxacin also has a potential for a serious drug interaction with NSAIDS. In addition, the package insert cautions that prothrombin time should be closely monitored if moxifloxacin is concomitantly administered with warfarin.[51]
A potentially serious drug interaction is the combination of corticosteroids and moxifloxacin, as this combination increases tendon toxicity, which has potential to result in tendonitis and disability.[52]
Overdose
"In the event of acute overdose, the stomach should be emptied and adequate hydration maintained. ECG monitoring is recommended due to the possibility of QT interval prolongation. The patient should be carefully observed and given supportive treatment. The administration of activated charcoal as soon as possible after oral overdose may prevent excessive increase of systemic moxifloxacin exposure. About 3% and 9% of the dose of moxifloxacin, as well as about 2% and 4.5% of its glucuronide metabolite are removed by continuous ambulatory peritoneal dialysis and hemodialysis, respectively."(sic) Quoting from the 12/29/2008 package insert for Avelox [39]
Mechanism of action
Moxifloxacin is a broad-spectrum antibiotic that is active against both Gram-positive and Gram-negative bacteria. It functions by inhibiting DNA gyrase, a type II topoisomerase, and topoisomerase IV,[53] enzymes necessary to separate bacterial DNA, thereby inhibiting cell replication.
This mechanism can also affect mammalian cell replication. In particular, some congeners of this drug family (for example those that contain the C-8 fluorine),[54] display high activity not only against bacterial topoisomerases, but also against eukaryotic topoisomerases and are toxic to cultured mammalian cells and in vivo tumor models.[55] Although quinolones are highly toxic to mammalian cells in culture, its mechanism of cytotoxic action is not known. Quinolone-induced DNA damage was first reported in 1986 (Hussy et al.).[56]
Recent studies have demonstrated a correlation between mammalian cell cytotoxicity of the quinolones and the induction of micronuclei.[57][58][59][60] As such, some fluoroquinolones, including moxifloxacin, may cause injury to the chromosome of eukaryotic cells.[61][62][63][64][65][66]
There continues to be considerable debate as to whether or not this DNA damage is to be considered one of the mechanisms of action concerning the severe adverse reactions experienced by some patients following fluoroquinolone therapy.[55][67][68]
Pharmacology
"Moxifloxacin, a fluoroquinolone, is available as the monohydrochloride salt of 13 1-cyclopropyl-7-[(S,S)-2,8-diazabicyclo[4.3.0]non-8-yl]-6-fluoro-8-methoxy-1,4-dihydro-4-oxo-3 14 quinoline carboxylic acid. It is a slightly yellow to yellow crystalline substance with a molecular 15 weight of 437.9. Its empirical formula is C21H24FN3O4 *HCl." Quoting from the 12/29/2008 package insert for Avelox [39]
There are a number of endogenous compounds that have been reported to be affected by moxifloxacin as inhibitors, alteraters, and depletors. See the latest package insert for Ciprofloxacin for additional details.
Pharmacokinetics
Approximately 52% of an oral or intravenous dose of moxifloxacin is metabolized via glucuronide and sulfate conjugation. The cytochrome P450 system is not involved in moxifloxacin metabolism, and is not affected by moxifloxacin. The sulfate conjugate (M1) accounts for approximately 38% of the dose, and is eliminated primarily in the feces. Approximately 14% of an oral or intravenous dose is converted to a glucuronide conjugate (M2), which is excreted exclusively in the urine. Peak plasma concentrations of M2 are approximately 40% those of the parent drug, while plasma concentrations of M1 are, in general, less than 10% those of moxifloxacin.[39]
In vitro studies with cytochrome (CYP) P450 enzymes indicate that moxifloxacin does not inhibit 80 CYP3A4, CYP2D6, CYP2C9, CYP2C19, or CYP1A2, suggesting that moxifloxacin is unlikely to alter the pharmacokinetics of drugs metabolized by these enzymes.[39]
The pharmacokinetics of moxifloxacin in pediatric subjects have not been studied.[39]
The half-life of moxifloxacin is 11.5-15.6 hours (single-dose, oral).[69] Approximately 45% of an oral or intravenous dose of moxifloxacin is excreted as unchanged drug (~20% in urine and ~25% in feces). A total of 96% ± 4% of an oral dose is excreted as either unchanged drug or known metabolites. The mean (± SD) apparent total body clearance and renal clearance are 12 ± 2 L/hr and 2.6 ± 0.5 L/hr, respectively.[69] The CSF penetration of moxifloxacin is 70% to 80% in patients with meningitis.[70]
Susceptible bacteria
A broad spectrum of bacteria is susceptible including, but not limited to:
- Staphylococcus aureus
- Staphylococcus epidermidis
- Streptococcus pneumoniae
- Haemophilus influenzae
- Klebsiella spp.
- Moraxella catarrhalis
- Enterobacter spp.
- Mycobacterium spp.
- Bacillus anthracis
History
Moxifloxacin was first patented (United States patent) in 1991[1] by Bayer A.G., and again in 1997.[71] Avelox was subsequently approved by the U.S. Food and Drug Administration (FDA) for use in the United States in 1999 to treat specific bacterial infections.[2] Ranking 140th within the top 200 prescribed drugs in the United States for 2007[72] moxifloxacin, in the same manner as ciprofloxacin, has proven to be a blockbuster drug for Bayer A. G., generating billions of dollars in additional revenue. In 2007 alone, Avelox generated sales of $697.3 million dollars worldwide.[28]
However, there were serious questions raised within the NDA (New Drug Application, 1999) regarding the safety and efficacy of moxifloxacin, which now appears to threaten its continuing viability. Of particular concern is moxifloxacin-induced cardiac arrhythmias. The Medical Review Officer stated that "Moxifloxacin clearly prolongs QTc intervals in a concentration-related manner and, as a result, puts patients at risk for developing malignant arrhythmias. The data provided by the sponsor did not include all patients treated, about 90% were excluded, and ECG were obtained as late as 6 hours after the drug intake (peak concentration is about 2 hours). So the sponsor's argument that moxifloxacin is safe because it causes only a small increase in QTc is flawed. What was shown in the database is that there are examples of patients on moxifloxacin with changes in QTc intervals greater than 80 msec over baseline with resulting QTc intervals above 500 msec.... Moxifloxacin raises the QTc interval in a concentration-related manner and, therefore, has the potential to cause malignant ventricular arrhythmias, including torsade de pointes, and death.... In summary, it is hard to justify approving this agent as a first-line therapy for non-life-threatening infections in which there are a plethora of treatment choices.(sic)"[73] The FDA approved moxifloxacin over the objections of the members of the FDA advisory committee,[74] as well as the medical review officer (noted above), even though the studies submitted with the NDA failed to show any advantage over the comprators presented in a number of instances.
Since the approval of moxifloxacin in 1999, there has been numerous upgrades to the warnings sections of the package inserts, as well as three Dear Doctor Letters issued by the manufacturer over the past year.[4][6][7] The latest involving the very issues raised with the NDA, ten years ago. Within the 2009 "Rote-Hand-Brief" German officials warned German physicians of the associated risk of cardiac arrhythmia in women and older patients.[4] At its July 2008 meeting, the agency's Committee for Medicinal Products for Human Use (CHMP) recommended restricting the use of moxifloxacin due to safety concerns, mainly related to an increased risk of adverse hepatic reactions.[28]
Nevertheless, moxifloxacin continues to be aggressively marketed by Bayer. Bayer makes about $500 million a year from moxifloxacin, which it sells in the United States as Avelox and elsewhere as Avalox, Avelon, Megaxin, Actira, and Izilox.[75]
Patent
A United States patent application was made on June 30, 1989 for Avelox (moxifloxacin hydrochloride),[1] (Bayer A.G. being the assignee), which was subsequently approved on February 5, 1991. This patent was scheduled to expire on June 30, 2009. However, this patent was extended for an additional two and one half years on September 16, 2004, and as such is not expected to expire until 2012.[76] Moxifloxacin was subsequently (ten years later) approved by the U.S. Food and Drug Administration (FDA) for use in the United States in 1999. There have been at least four additional United States patents filed regarding moxifloxacin hydrochloride since the 1989 United States application,[71][77] as well as patents outside of the USA.
Additional regulatory history
6/12/2002 Changes made to minimize the impact of warnings concerning adverse reactions.[78]
6/26/2003 New Zealand Pharmacovigilance warns of moxifloxacin induced respiratory insufficiency.[79]
10/6/2003 Changes made to minimize the impact of post marketing reports as well as the risk of tendon injuries.[80]
12/29/2008 Addition of numerous adverse reactions associated with the use of moxifloxacin.[81]
04/27/2009 Issuance of a Medication Guide and revisions to include new safety information including the addition of the Black Box Warning to the Medication Guide. The FDA had determined that Moxifloxacin poses a serious and significant public health concern, requiring the distribution of a Medication Guide.[82]
06/24/2009 Updating of the carton and container labels to include a statement to let dispensers know that a Medication Guide must be dispensed with the product.(emphasis added)[83]
History of the boxed warnings
Musculoskeletal disorders attributed to use of quinolone antibiotics were first reported in the medical literature in 1972, as an adverse reaction to nalidixic acid.[84] Rheumatic disease after use of a fluoroquinolone (norfloxacin) was first reported eleven years later.[85] In a 1995 letter published in the New England Journal of Medicine, representatives of the U.S. Food and Drug Administration (FDA) stated that the agency would "update the labeling [package insert] for all marketed fluoroquinolones to include a warning about the possibility of tendon rupture."[86]
By August 1996, the FDA had not taken action, and the consumer advocacy group Public Citizen filed a petition with the FDA, prompting the agency to act.[87] Two months later, the FDA published an alert in the FDA Medical Bulletin and requested that fluoroquinolone package inserts be amended to include information on this risk.[88]
In 2005, the Illinois Attorney General filed a petition with the FDA seeking Boxed Warnings and "Dear Doctor" letters emphasizing the risk of tendon rupture; the FDA responded that it had not yet been able to reach a decision on the matter.[89] In 2006, Public Citizen, supported by the Illinois Attorney General, renewed its demand of ten years prior for a Boxed Warning.[89][90] In January 2008, Public Citizen filed suit to compel the FDA to respond to their 2006 petition.[91][92] On July 7, the FDA ordered the makers of systemic-use fluoroquinolones to add a Boxed Warning regarding tendon rupture, and to develop a Medication Guide for patients.[93] The package inserts for Cipro (ciprofloxacin), Avelox (moxifloxacin), Proquin XR, Factive (gemifloxacin), Floxin (ofloxacin), Noroxin (norfloxacin) and Levaquin (levofloxacin) were amended on September 8, 2008 to include these new warnings.[94][95] Bayer, which manufactures Cipro, Avelox and Proquin XR, issued a Dear Healthcare Professional letter on October 22 concerning these changes.[96] Ortho-McNeil, the manufacturers of Levaquin, issued a similar letter in November.[97] through the Health Care Notification Network, a registration-only website that distributes drug alerts to licensed healthcare professionals.
Review of the FDA website indicates that the generic versions of the fluoroquinolones have not been updated to include this Boxed Warning as of February 2009. And there are numerous reports that this information has not been disseminated to the pharmacist, the products continue to contain the previous labels that are absent of this warning, and the Medication Guide has not been made available to the pharmacist or physician for distribution.
FDA warning letters
The manufacturers of Avelox (moxiflocaxin – Bayer A. G.) have received warning letters from the FDA regarding false advertising, promotion of unapproved uses, and failure to provide adequate warnings within their promotional materials:
December 15, 1999 Lack of fair balance and presentation of misleading safety information, promotion of unapproved uses, unsubstantiated superiority claims.[98]
June 21, 2001 Promotion of unapproved uses.[99]
Antibiotic misuse and bacterial resistance
See also: Antibiotic misuse and Antibiotic resistanceResistance to moxifloxacin and other fluoroquinolones may evolve rapidly, even during a course of treatment. Numerous pathogens, including Staphylococcus aureus, enterococci, and Streptococcus pyogenes now exhibit resistance worldwide.[100] Widespread veterinary usage of the fluoroquinolones, in particular in Europe, has been implicated.[101]
The ever-increasing bacterial resistance to moxifloxacin, (which is a major concern) together with an unacceptable safety profile, may very well threaten its future viability to treat serious and life-threatening bacterial infections. Years ago, the FDA had added warnings regarding the proper use of these drugs within the package inserts to combat such antibiotic abuse. Advising physicians that moxifloxacin: "...should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria....'"(See the monographs for this class)
Under normal circumstances, moxifloxacin and other fluoroquinolone drugs would be used only in patients having failed at least one prior therapy, reserved for the use in patients seriously ill and soon requiring immediate hospitalization.[102] Though considered to be a very important and necessary drug required to treat severe and life-threatening bacterial infections, the associated scripting abuse of moxifloxacin remains unchecked, which has contributed to the problem of bacterial resistance. The overuse of antibiotics, such as happens with children suffering from otitis media, has given rise to a breed of super-bacteria that are resistant to antibiotics entirely.[103]
For example, the use of the fuoroquinolones had increased three-fold in an emergency room environment in the United States between 1995 and 2002, while the use of safer alternatives such as macrolides declined significantly.[104][105]
Fluoroquinolones had become the most commonly prescribed class of antibiotics to adults in 2002. Nearly half (42%) of these prescriptions were for conditions not approved by the FDA, such as acute bronchitis, otitis media, and acute upper respiratory tract infection, according to a study that was supported in part by the Agency for Healthcare Research and Quality.[105][106] In addition, they are commonly prescribed for medical conditions that are not even bacterial to begin with, such as viral infections, or those to which no proven benefit exist.
Resistance to moxifloxacin arises in Mycobacterium tuberculosis if moxifloxacin is used alone instead of in combination with other anti-TB drugs,[107] which appears to be the explanation for the appearance of moxifloxacin resistance in newly diagnosed TB patients in Baltimore[108] and in Taiwan.[109] Of concern is that the development of resistance can appear in as short a time as seven days.[109] This calls into question the first-line use of moxifloxacin and other respiratory quinolones as first-line therapy for the treatment of community-acquired pneumonia in populations where TB is still endemic.
Social and economic impact
Main article: Quinolone#Social and economic impactSee also: Levofloxacin#Social and economic impact- Generic equivalents
In 2007, the U.S. District Court for the District of Delaware held that two Bayer patents on Avelox (moxifloxacin hydrochloride) are valid and enforceable, and infringed by Dr. Reddy's ANDA for a generic version of Avelox.[110][111] The district court sided with Bayer, citing the Federal Circuit's prior decision in Takeda v. Alphapharm [112] as "affirming the district court's finding that defendant failed to prove a prima facie case of obviousness where the prior art disclosed a broad selection of compounds, any one of which could have been selected as a lead compound for further investigation, and defendant did not prove that the prior art would have led to the selection of the particular compound singled out by defendant." According to Bayer's press release[110] announcing the court's decision, it was noted that Teva had also challenged the validity of the same Bayer patents at issue in the Dr. Reddy's case. Within Bayer's first quarter 2008 stockholder's newsletter[113] Bayer stated that they had reached an agreement with Teva Pharmaceuticals USA, Inc., the adverse party, to settle their patent litigation with regard to the two Bayer patents. Under the settlement terms agreed upon, Teva would obtain a license to sell its generic moxifloxacin tablet product in the U.S. shortly before the second of the two Bayer patents expires in March 2014.
- Economic impact: adverse reactions:
The adverse drug reaction profile of moxifloxacin and other fluoroquinolone drugs has spawned a grass-roots movement of those so affected to lobby for Black Box Warnings and Dear Doctor Letters as well as the petitioning of the FDA for the removal of some fluoroquinolone drugs from clinical practice.[114][115][116][117][118][119][120][121]
References
- ^ a b c http://fqresearch.org/pdf_files/avelox_patent_us.pdf
- ^ a b "Details for NDA:021085". DrugPatentWatch. http://www.drugpatentwatch.com/premium/preview/NDA/index.php?021085. Retrieved 17 July 2009.
- ^ a b Clodagh Sheehy (2 August 2008). "Warning over two types of antibiotic". Republic of Ireland. http://www.herald.ie/national-news/warning-over-two-types-of-antibiotic-1445498.html. Retrieved 17 July 2009.
- ^ a b c d e f g Bayer Schering Pharma (February 2008). "IMPORTANT INFORMATION REGARDING SERIOUS ADVERSE REACTIONS AND SAFETY MEASURES" (PDF). United Kingdom: Medicines and Healthcare products Regulatory Agency. http://www.mhra.gov.uk/home/groups/pl-p/documents/websiteresources/con014103.pdf. Retrieved 17 July 2009.
- ^ "Drug card for Moxifloxacin". Canada: DrugBank. 19 February 2009. http://www.drugbank.ca/drugs/DB00218. Retrieved 17 July 2009.
- ^ a b c "IMPORTANT DRUG WARNING" (PDF). 22 October 2008. http://www.rtialert.com/pdf/dhpl.pdf. Retrieved 17 July 2009.[dead link]
- ^ a b c d e Bayer Vital GmbH (19 January 2009). "WICHTIGE INFORMATIONEN ÜBER EINSCHRÄNKUNG DER INDIKATIONEN UND NEUE SEHR SELTENE UNERWÜNSCHTE WIRKUNGEN" (in German) (PDF). Germany: akdae. http://www.akdae.de/20/40/20090119.pdf. Retrieved 17 July 2009.
- ^ http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021085s047,021277s041lbl.pdf
- ^ "Avelox". The American Society of Health-System Pharmacists. http://www.drugs.com/monograph/avelox.html. Retrieved 3 April 2011.
- ^ http://www.accessdata.fda.gov/drugsatfda_docs/appletter/1999/21085ltr.pdf
- ^ a b c "EU agency recommends restricting moxifloxacin use". Reuters. 24 July 2008. http://uk.reuters.com/article/governmentFilingsNews/idUKL2453307820080724.
- ^ a b c Bayer Vital GmbH (19 January 2009). "WICHTIGE INFORMATIONEN ÜBER EINSCHRÄNKUNG DER INDIKATIONEN UND NEUE SEHR SELTENE UNERWÜNSCHTE WIRKUNGEN" (in German) (PDF). Germany: akdae. http://www.akdae.de/20/40/20090119.pdf. Retrieved on 17 July 2009.
- ^ http://www.accessdata.fda.gov/drugsatfda_docs/nda/2001/21-085S010_Avelox_Approv.pdf.
- ^ ^ http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2004/21085se1-022,21277se1-017ltr.pdf.
- ^ http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2005/021085s026,021277s022ltr.pdf.
- ^ ^ http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2005/021085s027,029,021277s024,025ltr.pdf.
- ^ European Medicines Agency (24 July 2008). "European Medicines Agency recommends restricting the use of oral moxifloxacin-containing medicines" (PDF). http://www.emea.europa.eu/pdfs/human/press/pr/38292708en.pdf. Retrieved 20 July 2009.
- ^ a b "SYNOPSIS". USA. http://download.veritasmedicine.com/PDF/CR002392_CSR.pdf. Retrieved 29 January 2009.
- ^ a b Karande SC, Kshirsagar NA (February 1992). "Adverse drug reaction monitoring of ciprofloxacin in pediatric practice". Indian Pediatr 29 (2): 181–8. PMID 1592498.
- ^ Dolui SK, Das M, Hazra A (2007). "Ofloxacin-induced reversible arthropathy in a child". Journal of Postgraduate Medicine 53 (2): 144–5. doi:10.4103/0022-3859.32220. PMID 17495385.
- ^ "Center for drug evaluation and research Application number 21-598" (PDF). USA: FDA. 15 April 2005. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2003/21-598_Vigamox_approv.PDF. Retrieved 21 July 2009.
- ^ Renata Albrecht (28 July 2004). "NDA 21-085/S-024, NDA 21-277/S-019" (PDF). Center for Drug Evaluation and Research. USA: FDA. http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2004/21277s019,21085s024ltr.pdf. Retrieved 31 July 2009.
- ^ Renata Albrecht (31 May 2007). "NDA 21-085/S-036, NDA 21-277/S-030" (PDF). Center for Drug Evaluation and Research. USA: FDA. http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2007/021085s036,021277s030ltr.pdf. Retrieved 31 July 2009.
- ^ Renata Albrecht (15 February 2008). "NDA 21-085/S-038, NDA 21-277/S-031" (PDF). Division of Special Pathogen and Transplant Products. USA: FDA. http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2008/021085s038ltr.pdf. Retrieved 31 July 2009.
- ^ DEPARTMENT OF HEALTH & HUMAN SERVICES (28 February 2008). "NDA 21-085/S-014, S-015, S-017" (PDF). USA: Food and Drug Administration. http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2003/21085slr014se1-015slr017,21277slr006se1-007slr009ltr.pdf. Retrieved 17 July 2009.
- ^ Nea Zealand Government (26 June 2003). "Adverse Reaction Reporting and IMMP". New Zealand: Medsafe. http://www.medsafe.govt.nz/Profs/adverse/Minutes114.htm. Retrieved 30 January 2009.
- ^ Bayer Pharmaceuticals (22 October 2008). "IMPORTANT DRUG WARNING" (PDF). USA. http://www.fqresearch.org/pdf_files/cipro_avelox-proquin_10_22_08_dear_doctor_letter.pdf. Retrieved 31 July 2009.
- ^ a b c "EU agency recommends restricting moxifloxacin use". Reuters. July 24, 2008. http://www.reuters.com/article/rbssHealthcareNews/idUSL2453307820080724. Retrieved 21 July 2009.
- ^ Renata Albrecht (3 October 2008). "NDA 21-085/S-040, NDA 21-277/S-034" (PDF). Center for Drug Evaluation and Research. USA: FDA. http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2008/021085s040,%20021277s034ltr.pdf. Retrieved 31 July 2009.
- ^ Updated Labeling for Antibiotic Avelox (Moxifloxacin) Regarding Risk of Severe Liver Injury / Information Update: 2010-42 For immediate release March 22, 2010 http://www.hc-sc.gc.ca/ahc-asc/media/advisories-avis/_2010/2010_42-eng.php
- ^ Saint F, Gueguen G, Biserte J, Fontaine C, Mazeman E (September 2000). "[Rupture of the patellar ligament one month after treatment with fluoroquinolone"] (in French). Rev Chir Orthop Reparatrice Appar Mot 86 (5): 495–7. PMID 10970974. http://www.masson.fr/masson/MDOI-RCO-09-2000-86-5-0035-1040-101019-ART7.
- ^ http://www.fda.gov/cder/foi/nda/99/21-085_Avelox.htm
- ^ Anti-Infective Drugs Advisory Committee 67th Meeting (Thursday, October 21, 1999) www.fqresearch.org/pdf_files/67th_fda_meeting.pdf
- ^ http://fqresearch.org/ftrf_drug_avelox.htm
- ^ Gangopadhyay N, Daniell M, Weih L, Taylor HR (April 2000). "Fluoroquinolone and fortified antibiotics for treating bacterial corneal ulcers". Br J Ophthalmol 84 (4): 378–84. doi:10.1136/bjo.84.4.378. PMC 1723447. PMID 10729294. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1723447.
- ^ Walter K, Tyler ME (August 2006). "Severe corneal toxicity after topical fluoroquinolone therapy: report of two cases". Cornea 25 (7): 855–7. doi:10.1097/01.ico.0000224642.43601.14. PMID 17068466.
- ^ a b Susan Blank; Julia Schillinger (14 May 2004). "DOHMH ALERT #8:Fluoroquinolone-resistant gonorrhea, NYC". USA: New York County Medical Society. http://www.nycms.org/article_view.php3?view=947&part=1. Retrieved 22 July 2009.
- ^ Centers for Disease Control and Prevention (CDC) (October 1995). "Fluoroquinolone resistance in Neisseria gonorrhoeae—Colorado and Washington, 1995". MMWR Morb Mortal Wkly Rep. 44 (41): 761–4. PMID 7565558. http://www.cdc.gov/mmwr/preview/mmwrhtml/00039305.htm.
- ^ a b c d e f g Bayer (December 2008). "AVELOX (moxifloxacin hydrochloride) Tablets AVELOX I.V. (moxifloxacin hydrochloride in sodium chloride injection)" (PDF). USA: FDA. p. 19. http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021085s039,021277s033lbl.pdf. Retrieved 02 Nov 2010.
- ^ a b c "Moxifloxacin". USA: University of Maryland Medical Center. 2009. http://www.umm.edu/altmed/drugs/moxifloxacin-088848.htm. Retrieved 22 July 2009.
- ^ Shin HC, Kim JC, Chung MK (September 2003). "Fetal and maternal tissue distribution of the new fluoroquinolone DW-116 in pregnant rats". Comp. Biochem. Physiol. C Toxicol. Pharmacol. 136 (1): 95–102. doi:10.1016/j.cca.2003.08.004. PMID 14522602.
- ^ Dan M, Weidekamm E, Sagiv R, Portmann R, Zakut H (February 1993). "Penetration of fleroxacin into breast milk and pharmacokinetics in lactating women" (PDF). Antimicrob. Agents Chemother. 37 (2): 293–6. PMC 187655. PMID 8452360. http://aac.asm.org/cgi/reprint/37/2/293.pdf.
- ^ Noel GJ, Bradley JS, Kauffman RE (October 2007). "Comparative safety profile of levofloxacin in 2523 children with a focus on four specific musculoskeletal disorders". Pediatr. Infect. Dis. J. 26 (10): 879–91. doi:10.1097/INF.0b013e3180cbd382. PMID 17901792.
- ^ a b Joette Meyer; Renata Albrecht (16 March 2004). "Division of Special Pathogen and Immunologic Drug Products Summary of Clinical Review of Studies Submitted in Response to a Pediatric Written Request" (PDF). USA: FDA. http://www.fda.gov/ohrms/dockets/AC/05/briefing/2005-4152b1_03_03_Cipro%20medical.pdf. Retrieved 22 July 2009.
- ^ R.W. Johnson (24 April 2007). "Levaquin (Levofloxacin) NDA 20634 Approved: 12/20/1996". USA: FDA. http://www.accessdata.fda.gov/drugsatfda_docs/nda/96/020634_levaquin_toc.cfm. Retrieved 22 July 2009.
- ^ "SYNOPSIS". USA. http://download.veritasmedicine.com/PDF/CR002389_CSR.pdf. Retrieved 29 January 2009.
- ^ Elbe DH, Chang SW (February 2005). "Moxifloxacin-warfarin interaction: a series of five case reports". Ann Pharmacother 39 (2): 361–4. doi:10.1345/aph.1E179. PMID 15632222. http://www.theannals.com/cgi/content/abstract/39/2/361.
- ^ a b Karen E. Brown. "Top Ten Dangerous Drug Interactions in Long-Term Care". Medication Management Project. http://www.scoup.net/m3project/topten/#10. Retrieved 1 August 2009.
- ^ Stass HH (June 29-July 3, 1997). "Bay 12-8039 (BA) does not interact with theophylline (TH)". Presented at the 20th International Congress of Chemotherapy (Sidney, Australia).
- ^ http://cme.medscape.com/viewarticle/409517_8
- ^ http://69.20.19.211/cder/warn/dec99/wl121599.pdf
- ^ Renata Albrecht (16 May 2002). "NDA 21-085/S-012" (PDF). USA: Food and Drug Administration. http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2002/21-085s012ltr.pdf. Retrieved 17 July 2009.
- ^ Drlica K, Zhao X (1 September 1997). "DNA gyrase, topoisomerase IV, and the 4-quinolones". Microbiol Mol Biol Rev. 61 (3): 377–92. PMC 232616. PMID 9293187. http://mmbr.asm.org/cgi/pmidlookup?view=long&pmid=9293187.
- ^ Robinson MJ, Martin BA, Gootz TD, McGuirk PR, Osheroff N (April 1992). "Effects of novel fluoroquinolones on the catalytic activities of eukaryotic topoisomerase II: Influence of the C-8 fluorine group" (PDF). Antimicrob. Agents Chemother. 36 (4): 751–6. PMC 189387. PMID 1323952. http://aac.asm.org/cgi/reprint/36/4/751.pdf.
- ^ a b Sissi C, Palumbo M (November 2003). "The quinolone family: from antibacterial to anticancer agents". Curr Med Chem Anticancer Agents 3 (6): 439–50. doi:10.2174/1568011033482279. PMID 14529452. http://openurl.ingenta.com/content/nlm?genre=article&issn=1568-0118&volume=3&issue=6&spage=439&aulast=Sissi. "The present review focuses on the structural modifications responsible for the transformation of an antibacterial into an anticancer agent. Indeed, a distinctive feature of drugs based on the quinolone structure is their remarkable ability to target different type II topoisomerase enzymes. In particular, some congeners of this drug family display high activity not only against bacterial topoisomerases but also against eukaryotic topoisomerases and are toxic to cultured mammalian cells and in vivo tumor models."
- ^ Hussy P, Maass G, Tümmler B, Grosse F, Schomburg U (June 1986). "Effect of 4-quinolones and novobiocin on calf thymus DNA polymerase alpha primase complex, topoisomerases I and II, and growth of mammalian lymphoblasts". Antimicrob. Agents Chemother. 29 (6): 1073–8. PMC 180502. PMID 3015015. http://aac.asm.org/cgi/reprint/29/6/1073.pdf.
- ^ "Mutagenicity of norfloxacin and AM-833 in bacteria and mammalian cells". Rev. Infect. 1 (jstor.org) 10: S148–S149. 1988. http://www.jstor.org/pss/4454399.
- ^ Forsgren, A, Bredberg A, Pardee AB, Schlossman SF, Tedder TF (May 1987). "Effects of ciprofloxacin on eucaryotic pyrimidine nucleotide biosynthesis and cell growth.". Antimicrobial Agents and Chemotherapy 31 (5): 774–9. PMC 174831. PMID 3606077. http://aac.asm.org/cgi/pmidlookup?view=long&pmid=3606077.
- ^ Gootz TD, Barrett JF, Sutcliffe JA (January 1990). "Inhibitory effects of quinolone antibacterial agents on eucaryotic topoisomerases and related test systems". Antimicrob. Agents Chemother. 34 (1): 8–12. PMC 171510. PMID 2158274. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=171510.
- ^ Lawrence JW, Darkin-Rattray S, Xie F, Neims AH, Rowe TC (February 1993). "4-Quinolones cause a selective loss of mitochondrial DNA from mouse L1210 leukemia cells". J. Cell. Biochem. 51 (2): 165–74. doi:10.1002/jcb.240510208. PMID 8440750.
- ^ Elsea SH, Osheroff N, Nitiss JL (July 1992). "Cytotoxicity of quinolones toward eukaryotic cells. Identification of topoisomerase II as the primary cellular target for the quinolone CP-115,953 in yeast". J. Biol. Chem. 267 (19): 13150–3. PMID 1320012. http://www.jbc.org/cgi/reprint/267/19/13150.
- ^ Suto MJ, Domagala JM, Roland GE, Mailloux GB, Cohen MA (December 1992). "Fluoroquinolones: relationships between structural variations, mammalian cell cytotoxicity, and antimicrobial activity". J. Med. Chem. 35 (25): 4745–50. doi:10.1021/jm00103a013. PMID 1469702.
- ^ Enzmann H, Wiemann C, Ahr HJ, Schlüter G (April 1999). "Damage to mitochondrial DNA induced by the quinolone Bay y 3118 in embryonic turkey liver". Mutat. Res. 425 (2): 213–24. doi:10.1016/S0027-5107(99)00044-5. PMID 10216214.
- ^ Kashida Y, Sasaki YF, Ohsawa K (October 2002). "Mechanistic study on flumequine hepatocarcinogenicity focusing on DNA damage in mice". Toxicol. Sci. 69 (2): 317–21. doi:10.1093/toxsci/69.2.317. PMID 12377980. http://toxsci.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=12377980.
- ^ Thomas A, Tocher J, Edwards DI (May 1990). "Electrochemical characteristics of five quinolone drugs and their effect on DNA damage and repair in Escherichia coli". J. Antimicrob. Chemother. 25 (5): 733–44. doi:10.1093/jac/25.5.733. PMID 2165050. http://jac.oxfordjournals.org/cgi/reprint/25/5/733.
- ^ "Fluoroquinolones and Quinolones". The American Academy of Optometry (British Chapter). http://www.academy.org.uk/pharmacy/fluoroq.htm. Retrieved 29 January 2009.
- ^ Yaseen A. Al-Soud; Najim A. Al-Masoudi (2003). "A new class of dihaloquinolones bearing N'-aldehydoglycosylhydrazides, mercapto-1,2,4-triazole, oxadiazoline and a-amino ester precursors: synthesis and antimicrobial activity". J. Braz. Chem. Soc 14 (5). doi:10.1590/S0103-50532003000500014. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0103-50532003000500014&lng=es&nrm=iso&tlng=es. "Nevertheless, some quinolones cause injury to the chromosome of eukaryotic cells.21,22 These findings prompted us to optimize the substituent at C-3, by..."
- ^ Yaseen A. Al-Soud a and Najim A. Al-Masoudi (2003). "A New Class of Dihaloquinolones Bearing N’-Aldehydoglycosylhydrazides, Mercapto-1,2,4-triazole, Oxadiazoline and α-Amino Ester Precursors: Synthesis and Antimicrobial Activity". J. Braz. Chem. Soc 14 (5): 790–796. doi:10.1590/S0103-50532003000500014. http://jbcs.sbq.org.br/jbcs/2003/v14_n5/13-048-02.pdf. "Although the current quinolones are not considered to be potent inhibitors of eucaryotic topoisomerases, some effects on these and other enzymes involved with DNA replication have been observed"
- ^ a b "Drug card for Moxifloxacin (DB00218)". Canada: DrugBank. 19 February 2009. http://www.drugbank.ca/drugs/DB00218. Retrieved 3 August 2009.
- ^ Alffenaar J. W. C., van Altena R., Bökkerink H. J (2009). "Pharmacokinetics of moxifloxacin in cerebrospinal fluid and plasma in patients with tuberculous meningitis". Clinical Infectious Diseases 49 (7): 1080–2. doi:10.1086/605576. PMID 19712035.
- ^ a b "Inventors/Applicants". patentlens.net. 2006-10-03. http://www.patentlens.net/patentlens/search_ajax.cgi?patnum=US/7115744. Retrieved 17 July 2009.
- ^ Ed Lamb (1 May 2008). "Top 200 Prescription Drugs of 2007". Pharmacy Times. http://www.pharmacytimes.com/issues/articles/2008-05_003.asp. Retrieved 21 July 2009.
- ^ FDA (1999). "Avelox (Moxifloxacin Hydrochloride) Tablets". USA: Food and Drug Administration. http://www.accessdata.fda.gov/drugsatfda_docs/nda/99/21-085_Avelox.cfm. Retrieved 17 July 2009.
- ^ Food and Drug Administration (21st OCTOBER 1999). "ANTI-INFECTIVE DRUGS ADVISORY COMMITTEE 67TH MEETING". USA: Food and Drug Administration. http://www.fda.gov/ohrms/dockets/ac/99/transcpt/3558t2.rtf. Retrieved 17 July 2009.
- ^ Times, The New York (18 October 2005). "Bayer Offers New Antibiotic With Promise in Fight on TB". TB Alliance. http://www.tballiance.org/newscenter/view-innews.php?id=157. Retrieved 18 July 2009.
- ^ http://www.uspto.gov/web/offices/pac/dapp/opla/term/certs/4990517.pdf alternate link fqresearch.org/pdf_files/avelox_patent_ext.pdf
- ^ US 4990517 A (Feb, 1991) Petersen et al. See http://www.patentlens.net/patentlens/search_ajax.cgi?patnum=US+4990517#list US 5051509 A (Sep, 1991) Nagano et al. See http://www.patentlens.net/patentlens/search_ajax.cgi?patnum=US+5051509#list US 5059597 A (Oct, 1991) Petersen et al. See http://www.patentlens.net/patentlens/search_ajax.cgi?patnum=US+5059597#list US 5395944 A (Mar, 1995) Petersen et al. See http://www.patentlens.net/patentlens/search_ajax.cgi?patnum=US+5395944#list US 5416096 A (May, 1995) Petersen et al. See http://www.patentlens.net/patentlens/search_ajax.cgi?patnum=US+5416096#list
- ^ Renata Albrecht (12 June 2002). "NDA 21-085/S-006, S-007 - NDA 21-277/S-002" (PDF). USA: FDA. http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2002/21085s006ltr.pdf. Retrieved 12 August 2009.
- ^ THE 114TH MEDICINES ADVERSE REACTIONS COMMITTEE MEETING; Professor P. Ellis, Professor D.C.G. Skegg, Dr M. Rademaker, Dr F. McClure, Dr N. Rafter, Dr M. Tatley (26 June 2003). "Adverse Reaction Reporting and IMMP". New Zealand: Medsafe. http://www.medsafe.govt.nz/Profs/adverse/Minutes114.htm. Retrieved 12 August 2009.
- ^ Renata Albrecht, (6 October 2003). "NDA 21-085/S-019 - NDA 21-277/S-011" (PDF). USA: FDA. http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2003/21277slr011,21085slr019ltr.pdf. Retrieved 12 August 2009.
- ^ Renata Albrecht (6 October 2003). "NDA 21-085/S-039 NDA 21-277/S-033" (PDF). USA: FDA. http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2008/021085s039,%20021277s033ltr.pdf. Retrieved 12 August 2009.
- ^ Bayer HealthCare Pharmaceuticals Inc. (August 2008). "MEDICATION GUIDE AVELOX (AV-eh-locks) (moxifloxacin hydrochloride) Tablets AVELOX I.V. (AV-eh-locks) (moxifloxacin hydrochloride in sodium chloride injection)" (PDF). USA: FDA. http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/021085s042,021277s036lbl.pdf. Retrieved 12 August 2009.
- ^ "NDA 21-085/S-041 NDA 21-277/S-035" (PDF). USA: FDA. 24 June 2009. http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2009/021085s041,021277s035ltr.pdf. Retrieved 12 August 2009.
- ^ Bailey RR, Natale R, Linton AL (October 1972). "Nalidixic acid arthralgia". Can Med Assoc J 107 (7): 604 passim. PMC 1940945. PMID 4541768. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1940945.
- ^ Bailey RR, Kirk JA, Peddie BA (July 1983). "Norfloxacin-induced rheumatic disease". N Z Med J 96 (736): 590. PMID 6223241.
- ^ Szarfman A, Chen M, Blum MD (January 1995). "More on fluoroquinolone antibiotics and tendon rupture" (letter). N Engl J Med 332 (3): 193. doi:10.1056/NEJM199501193320319. PMID 7800023.
- ^ "Petition to Require a Warning on All Fluoroquinolone Antibiotics (HRG Publication #1399)". Public Citizen. August 1, 1996. http://www.citizen.org/publications/release.cfm?ID=6595. Retrieved on December 27, 2008.
- ^ "Reports of adverse events with fluoroquinolones". FDA Medical Bulletin 26 (3). October 1996. http://www.fda.gov/medbull/oct96/adverse.html.[dead link] Retrieved on December 27, 2008. alternate link: http://www.fqresearch.org/text_documents/FDA_Medical_Bulletin_1996.doc
- ^ a b "Madigan, Public Citizen, petition FDA for "black box" warning regarding potential adverse effects of certain popular antibiotics" (Press release). Office of the Illinois Attorney General. August 29, 2006. http://www.illinoisattorneygeneral.gov/pressroom/2006_08/20060829.html. Retrieved 2008-12-27. Full text of the 2005 petition and FDA response available from the Fluoroquinolone Toxicity Research Foundation, a U.S. consumer advocacy group.
- ^ "Public Citizen Petitions the FDA to Include a Black Box Warning on Fluoroquinolone Antibiotics (HRG Publication #1781)". Public Citizen. August 29, 2006. http://www.citizen.org/publications/release.cfm?ID=7453. Retrieved 2008-12-27.
- ^ "Public Citizen v. Food and Drug Administration (FDA) (Fluoroquinolone)". Public Citizen. January 3, 2008. http://www.citizen.org/litigation/forms/cases/CaseDetails.cfm?cID=444. Retrieved 2008-12-27.
- ^ Ravn, Karen (August 18, 2008). "Behind the FDA's ‘black box’ warnings". Los Angeles Times. http://articles.latimes.com/2008/aug/18/health/he-closer18. Retrieved 2008-12-27.
- ^ "FDA Requests Boxed Warnings on Fluoroquinolone Antimicrobial Drugs" (Press release). U.S. Food and Drug Administration. 2008-07-08. http://www.fda.gov/bbs/topics/NEWS/2008/NEW01858.html. Retrieved 2008-10-11.
- ^ "Drugs@FDA". USA: FDA. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm. Retrieved 12 August 2009.
- ^ FDA. "MedWatch: The FDA Safety Information and Adverse Event Reporting Program". USA: FDA. http://www.fda.gov/medwatch. Retrieved 12 August 2009.
- ^ MacCarthy, Paul (October 22, 2008). "Important Change in the Avelox (moxifloxacin hydrochloride) and Cipro (ciprofloxacin) Complete Prescribing Information – Addition of Boxed Warning and Medication Guide Regarding Tendinitis and Tendon Rupture". Bayer HealthCare Pharmaceuticals. http://www.cipro.com/html/pdf/dhpl.pdf. Retrieved 2008-12-27.
- ^ Rosenthal, Norman (November 2008). "Important Change in the LEVAQUIN (Ievofloxacin) Complete Prescribing Information -Addition of Boxed Warning and Medication Guide Regarding Tendinitis and Tendon Rupture". Ortho-McNeil Janssen Scientific Affairs, LLC. http://www.fqresearch.org/pdf_files/Levaquin_11_2008_ortho_mcneil_dear_dr_letter.pdf. Retrieved 2008-12-27.
- ^ FDA (15 December 1999). "NDA #21-085 Avelox (moxifloxacin hydrochloride) Tablets Macmis ID #8577" (PDF). USA: Food and Drug Administration. http://www.fda.gov/Cder/warn/dec99/wl121599.pdf. Retrieved 30 January 2009.[dead link] alternative link: http://www.fqresearch.org/pdf_files/avelox_warning_1999.pdf
- ^ James R. Rodgers (29 June 2001). "NDA #21-085 Avelox (moxifloxacin HCL) MACMIS ID #10160" (PDF). USA: FDA. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/EnforcementActivitiesbyFDA/WarningLettersandNoticeofViolationLetterstoPharmaceuticalCompanies/UCM166447.pdf. Retrieved 12 August 2009. alternate link: fqresearch.org/pdf_files/UCM166447.pdf
- ^ Jacobs MR (July 2005). "Antimicrobial Agents And Resistance--Fifth International Symposium". IDrugs 8 (7): 542–6. PMID 15973559.
- ^ FDA (16 July 1999). "Update On Extra-Label Use Of Fluoroquinolones". USA: FDA. http://www.fda.gov/AnimalVeterinary/NewsEvents/CVMUpdates/ucm127657.htm. Retrieved 12 August 2009.
- ^ "ALASKA MEDICAID PHARMACY AND THERAPEUTICS COMMITTEE" (PDF). Alaska, USA. 19 March 2004. http://www.hss.state.ak.us/dhcs/PDL/minutes_meetings_pdl/minutes_031904_pdl.pdf. Retrieved 12 August 2009.
- ^ Froom J, Culpepper L, Jacobs M (July 1997). "Antimicrobials for acute otitis media? A review from the International Primary Care Network". BMJ 315 (7100): 98–102. PMC 2127061. PMID 9240050. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2127061.
- ^ MacDougall C, Guglielmo BJ, Maselli J, Gonzales R (March 2005). "Antimicrobial drug prescribing for pneumonia in ambulatory care". Emerging Infect. Dis. 11 (3): 380–4. PMID 15757551. http://www.cdc.gov/ncidod/EID/vol11no03/04-0819.htm.
- ^ a b Linder JA, Huang ES, Steinman MA, Gonzales R, Stafford RS (March 2005). "Fluoroquinolone prescribing in the United States: 1995 to 2002". Am. J. Med. 118 (3): 259–68. doi:10.1016/j.amjmed.2004.09.015. PMID 15745724. http://linkinghub.elsevier.com/retrieve/pii/S0002-9343(04)00711-9.
- ^ K08 HS14563 and HS11313
- ^ Ginsburg AS, Sun R, Calamita H (2005). "Emergence of fluoroquinolone resistance in Mycobacterium tuberculosis during continuously dosed moxifloxacin monotherapy in a mouse model". Antimicrob Agents Chemother 49 (9): 3977–79. doi:10.1128/AAC.49.9.3977-3979.2005. PMC 1195434. PMID 16127087. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1195434.
- ^ Ginsburg AS, Hooper N, Parrish N (2003). "Fluoroquinolone resistance in patients with newly diagnosed tuberculosis". Clin Infect Dis 37 (11): 1448–52. doi:10.1086/379328. PMID 14614666.
- ^ a b Wang J-Y, Hsueh P-R, Jan I-S (2006). "Empirical treatment with a fluoroquinolone delays the treatment for tuberculosis and is associated with a poor prognosis in endemic areas". Thorax 61 (10): 903–8. doi:10.1136/thx.2005.056887. PMC 2104756. PMID 16809417. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2104756.
- ^ a b Bayer AG (6 November 2007). "Ruling in Bayer's favor over Avelox patents". Bayer. http://www.stockholders-newsletter-q3-07.bayer.com/en/avelox-patents.aspx. Retrieved 29 August 2009.
- ^ http://www.orangebookblog.com/Bayer_20v._20Dr._20Reddy_27s.pdf
- ^ "United States Court of Appeals for the Federal Circuit" (PDF). USA: uscourts.gov. 28 June 2007. http://www.cafc.uscourts.gov/opinions/06-1329.pdf. Retrieved 29 August 2009.[dead link]
- ^ Bayer AG (24 April 2008). "Risk Report". Bayer. http://www.stockholders-newsletter-q1-08.bayer.com/en/Risk-Report.aspx. Retrieved 29 August 2009.
- ^ In The United States District Court For The District Of Columbia Public Citizen, Inc. VS. Food And Drug Administration January 3, 2008
- ^ Office of the Attorney General State of Illinois Lisa Madigan Citizen Petition to Include a Black Box Warning on Fluoroquinolone Antibiotics May 18, 2005
- ^ Public Citizen's Petition to Include a Black Box Warning on Fluoroquinolone Antibiotics (HRG Publication #1781) August 29, 2006
- ^ Public Citizen's Petition to Require a Warning on All Fluoroquinolone Antibiotics (HRG Publication #1399) August 1, 1996
- ^ Public Citizen's Petition to Ban the Antibiotic Gatifloxacin (Tequin) (HRG Publication #1768)
- ^ Public Citizen's Petition to immediately ban the antibiotic Trovafloxacin (Trovan). (HRG Publication #1485) Date: June 3, 1999
- ^ Public Citizen's Petition to immediately stop the distribution of dangerous, misleading prescription drug information to the public. HRG Publication #1442 Date: June 9, 1998
- ^ June 2004, A petition To the United States Congress to immediately take action to protect consumers from the reckless and negligent abuses of the FDA and the following Pharmaceutical Companies: Bayer, Ortho-McNeill, Pfizer, Merck, Bristol-Myers Squibb, Sanofi Winthrop, Bertek Pharmaceuticals – Rhone-Poulenc Rorer and Barr. These companies manufacture and distribute fluoroquinolone antibiotics in the United States in a manner that fails to warn of serious adverse event risks, and downplays and fails to warn physicians of the serious risks associated with fluoroquinolone therapy.
Antibacterials: nucleic acid inhibitors (J01E, J01M) Antifolates
(inhibits
purine metabolism,
thereby inhibiting
DNA and RNA synthesis)Sulfonamides
(DHPS inhibitor)Other/ungroupedCombinationsTopoisomerase
inhibitors/
quinolones/
(inhibits
DNA replication)1st g.2nd g.Ciprofloxacin# • Enoxacin‡ • Fleroxacin‡ • Lomefloxacin • Nadifloxacin • Ofloxacin • Norfloxacin • Pefloxacin • Rufloxacin3rd g.4th g.Besifloxacin • Clinafloxacin† • Garenoxacin • Gemifloxacin • Moxifloxacin • Gatifloxacin‡ • Sitafloxacin • Trovafloxacin‡/Alatrofloxacin‡ • PrulifloxacinVet.Related (DG)Anaerobic DNA
inhibitorsNitrofuran derivativesRNA synthesis Categories:- Fluoroquinolone antibiotics
- Pyrrolopyridines
- Phenol ethers
- Cyclopropanes
Wikimedia Foundation. 2010.
