- Kinesin
-
A kinesin is a protein belonging to a class of motor proteins found in eukaryotic cells. Kinesins move along microtubule filaments, and are powered by the hydrolysis of ATP (thus kinesins are ATPases). The active movement of kinesins supports several cellular functions including mitosis, meiosis and transport of cellular cargo, such as in axonal transport. Most kinesins walk towards the plus end of a microtubule, which, in most cells, entails transporting cargo from the centre of the cell towards the periphery. This form of transport is known as anterograde transport.
Contents
The Kinesins
Kinesins were discovered as microtubule (MT)-based anterograde intracellular transport motors.[1] The founding member of this superfamily, kinesin-1, was isolated as a heterotetrameric fast axonal organelle transport motor consisting of 2 identical motor subunits (KHC) and 2 "light chains" (KLC) via microtubule affinity purification from neuronal cell extracts.[2] Subsequently a different, heterotrimeric plus-end-directed MT-based motor named kinesin-2, consisting of 2 distinct KHC-related motor subunits and an accessory "KAP" subunit, was purified from echinoderm egg/embryo extracts[3] and is best known for its role in transporting protein complexes (IFT particles) along axonemes during cilium biogenesis.[4] Molecular genetic and genomic approaches have led to the recognition that the kinesins form a diverse superfamily of motors that are responsible for multiple intracellular motility events in eukaryotic cells.[5][6][7][8] For example, the genomes of mammals encode more than 40 kinesin proteins,[9] organized into at least 14 families named kinesin-1 through kinesin-14.[10]
Structure
Overall structure
Members of the kinesin superfamily vary in shape but the prototypical kinesin-1 is a heterotetramer whose motor subunits (heavy chains or KHCs) form a protein dimer (molecule pair) that binds two light chains (KLCs).
The heavy chain of kinesin-1 comprises a globular head (the motor domain) at the amino terminal end connected via a short, flexible neck linker to the stalk – a long, central alpha-helical coiled-coil domain – that ends in a carboxy terminal tail domain which associates with the light-chains. The stalks of two KHCs intertwine to form a coiled-coil that directs dimerization of the two KHCs. In most cases transported cargo binds to the kinesin light chains, but in some cases cargo binds to the C-terminal domains of the heavy chains.[11]
Kinesin motor domain
Kinesin motor domain 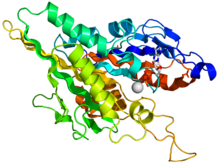
Crystallographic structure of the human kinesin motor domain depicted as a rainbow colored cartoon (N-terminus = blue, C-terminus = red) complexed with ADP (stick diagram, carbon = white, oxygen = red, nitrogen = blue, phosphorus = orange) and a magnesium ion (grey sphere).[12] Identifiers Symbol Kinesin motor domain Pfam PF00225 InterPro IPR001752 SMART SM00129 PROSITE PS50067 SCOP 1bg2 Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary The head is the signature of kinesin and its amino acid sequence is well conserved among various kinesins. Each head has two separate binding sites: one for the microtubule and the other for ATP. ATP binding and hydrolysis as well as ADP release change the conformation of the microtubule-binding domains and the orientation of the neck linker with respect to the head; this results in the motion of the kinesin. Several structural elements in the head, including a central beta-sheet domain and the Switch I and II domains, have been implicated as mediating the interactions between the two binding sites and the neck domain. Kinesins are related structurally to G proteins, which hydrolyze GTP instead of ATP. Several structural elements are shared between the two families, notably the Switch I and Switch II domains.
Cargo transport
In the cell, small molecules such as gases and glucose diffuse to where they are needed. Large molecules synthesised in the cell body, intracellular components such as vesicles, and organelles such as mitochondria are too large (and the cytosol too crowded) to diffuse to their destinations. Motor proteins fulfill the role of transporting large cargo about the cell to their required destinations. Kinesins are motor proteins that transport such cargo by walking unidirectionally along microtubule tracks hydrolysing one molecule of adenosine triphosphate (ATP) at each step.[13] It was thought that ATP hydrolysis powered each step, the energy released propelling the head forwards to the next binding site.[14] However, it has been proposed that the head diffuses forward and the force of binding to the microtubule is what pulls the cargo along.[15]
There is significant evidence that cargoes in-vivo are transported by multiple motors.[16][17][18][19]
Direction of motion
Motor proteins travel in a specific direction along a microtubule. This is because the microtubule is polar and the heads only bind to the microtubule in one orientation, while ATP binding gives each step its direction through a process known as neck linker zippering.[20]
Most kinesins walk towards the plus end of a microtubule which, in most cells, entails transporting cargo from the centre of the cell towards the periphery. This form of transport is known as anterograde transport. Kinesin-14 family proteins, such as Drosophila NCD, budding yeast KAR3, and Arabidopsis ATK5, walk in the opposite direction, toward microtubule minus ends.[21]
A different type of motor protein known as dyneins, move towards the minus end of the microtubule. Thus they transport cargo from the periphery of the cell towards the centre, for example from the terminal buttons of a neuronal axon to the cell body (soma). This is known as retrograde transport.
Proposed mechanisms of movement
Kinesin accomplishes transport by "walking" along a microtubule. Two mechanisms have been proposed to account for this movement.
- In the "hand-over-hand" mechanism, the kinesin heads step past one another, alternating the lead position.
- In the "inchworm" mechanism, one kinesin head always leads, moving forward a step before the trailing head catches up.
Despite some remaining controversy, mounting experimental evidence points towards the hand-over-hand mechanism as being more likely.[22][23]
ATP binding and hydrolysis cause kinesin to travel via a "seesaw mechanism" about a pivot point.[24]
Theoretical modeling of kinesin
A number of theoretical models of the molecular motor protein Kinesin have been proposed.[25][26][27] Many challenges are encountered in theoretical investigations given the remaining uncertainties about the roles of protein structures, precise way energy from ATP is transformed into mechanical work, and the roles played by thermal fluctuations. This is a rather active area of research. There is a need especially for approaches which better make a link with the molecular architecture of the protein and data obtained from experimental investigations.
Kinesin and mitosis
In recent years, it has been found that microtubule-based molecular motors (including a number of kinesins) have a role in mitosis (cell division). Kinesins are important for proper spindle length and are involved in sliding microtubules apart within the spindle during prometaphase and metaphase, as well as depolymerizing microtubule minus ends at centrosomes during anaphase.[28] Specifically, Kinesin-5 family proteins act within the spindle to slide microtubules apart, while the Kinesin 13 family act to depolymerize microtubules.
Kinesin Superfamily members
Human kinesin superfamily members include the following proteins, which in the standardized nomenclature developed by the community of kinesin researchers, are organized into 14 families named kinesin-1 through kinesin-14:[10]
- 1A – KIF1A, 1B – KIF1B = kinesin-3
- 2A – KIF2A, 2C – KIF2C = kinesin-13
- 3B – KIF3B, 3C – KIF3C = kinesin-2
- 4A – KIF4A, 4B – KIF4B = kinesin-4
- 5A – KIF5A, 5B – KIF5B, 5C – KIF5C = kinesin-1
- 6 – KIF6 = kinesin-9
- 7 – KIF7 = kinesin-4
- 9 – KIF9 = kinesin-9
- 11 – KIF11 = kinesin-5
- 12 – KIF12 = kinesin-12
- 13A – KIF13A, 13B – KIF13B = kinesin-3
- 14 – KIF14 = kinesin-3
- 15 – KIF15 = kinesin-12
- 16B – KIF16B = kinesin-3
- 17 – KIF17 = kinesin-2
- 18A – KIF18A, 18B – KIF18B = kinesin-8
- 19 – KIF19 = kinesin-8
- 20A – KIF20A, 20B – KIF20B = kinesin-6
- 21A – KIF21A, 21B – KIF21B = kinesin-4
- 22 – KIF22 = kinesin-10
- 23 – KIF23 = kinesin-6
- 24 – KIF24 = kinesin-13
- 25 – KIF25 = kinesin-14
- 26A – KIF26A, 26B – KIF26B = kinesin-11
- 27 – KIF27 = kinesin-4
- C1 – KIFC1, C2 – KIFC2, C3 – KIFC3 = kinesin-14
kinesin-1 light chains:
kinesin-2 associated protein:
- KAP-1, KAP3 or KIFAP3
See also
- Axoplasmic transport
- Dynein
- Intraflagellar transport along cilia
- Kinesin 8
- Kinesin 13
- KRP
- Molecular motors
- Transport by Multiple Kinesin
References
- ^ Vale RD (February 2003). "The molecular motor toolbox for intracellular transport". Cell 112 (4): 467–80. PMID 12600311.
- ^ Vale RD, Reese TS, Sheetz MP (August 1985). "Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility". Cell 42 (1): 39–50. doi:10.1016/S0092-8674(85)80099-4. PMC 2851632. PMID 3926325. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2851632.
- ^ Cole DG, Chinn SW, Wedaman KP, Hall K, Vuong T, Scholey JM (November 1993). "Novel heterotrimeric kinesin-related protein purified from sea urchin eggs". Nature 366 (6452): 268–70. doi:10.1038/366268a0. PMID 8232586.
- ^ Rosenbaum JL, Witman GB (November 2002). "Intraflagellar transport". Nat. Rev. Mol. Cell Biol. 3 (11): 813–25. doi:10.1038/nrm952. PMID 12415299.
- ^ Yang JT, Laymon RA, Goldstein LS (March 1989). "A three-domain structure of kinesin heavy chain revealed by DNA sequence and microtubule binding analyses". Cell 56 (5): 879–89. doi:10.1016/0092-8674(89)90692-2. PMID 2522352.
- ^ Aizawa H, Sekine Y, Takemura R, Zhang Z, Nangaku M, Hirokawa N (December 1992). "Kinesin family in murine central nervous system". J. Cell Biol. 119 (5): 1287–96. doi:10.1083/jcb.119.5.1287. PMC 2289715. PMID 1447303. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2289715.
- ^ Enos AP, Morris NR (March 1990). "Mutation of a gene that encodes a kinesin-like protein blocks nuclear division in A. nidulans". Cell 60 (6): 1019–27. doi:10.1016/0092-8674(90)90350-N. PMID 2138511.
- ^ Meluh PB, Rose MD (March 1990). "KAR3, a kinesin-related gene required for yeast nuclear fusion". Cell 60 (6): 1029–41. doi:10.1016/0092-8674(90)90351-E. PMID 2138512.
- ^ Hirokawa N, Noda Y, Tanaka Y, Niwa S (October 2009). "Kinesin superfamily motor proteins and intracellular transport". Nat. Rev. Mol. Cell Biol. 10 (10): 682–96. doi:10.1038/nrm2774. PMID 19773780.
- ^ a b Lawrence CJ, Dawe RK, Christie KR, Cleveland DW, Dawson SC, Endow SA, Goldstein LS, Goodson HV, Hirokawa N, Howard J, Malmberg RL, McIntosh JR, Miki H, Mitchison TJ, Okada Y, Reddy AS, Saxton WM, Schliwa M, Scholey JM, Vale RD, Walczak CE, Wordeman L (October 2004). "A standardized kinesin nomenclature". J. Cell Biol. 167 (1): 19–22. doi:10.1083/jcb.200408113. PMC 2041940. PMID 15479732. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2041940.
- ^ Hirokawa N, Pfister KK, Yorifuji H, Wagner MC, Brady ST, Bloom GS (March 1989). "Submolecular domains of bovine brain kinesin identified by electron microscopy and monoclonal antibody decoration". Cell 56 (5): 867–78. PMID 2522351.
- ^ PDB 1BG2; Kull FJ, Sablin EP, Lau R, Fletterick RJ, Vale RD (April 1996). "Crystal structure of the kinesin motor domain reveals a structural similarity to myosin". Nature 380 (6574): 550–5. Bibcode 1996Natur.380..550J. doi:10.1038/380550a0. PMC 2851642. PMID 8606779. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2851642.
- ^ Schnitzer MJ, Block SM (1997). "Kinesin hydrolyses one ATP per 8-nm step". Nature 388 (6640): 386–390. Bibcode 1997Natur.388..386S. doi:10.1038/41111. PMID 9237757.
- ^ Vale RD, Milligan RA (April 2000). "The way things move: looking under the hood of molecular motor proteins". Science 288 (5463): 88–95. Bibcode 2000Sci...288...88V. doi:10.1126/science.288.5463.88. PMID 10753125.
- ^ Mather WH, Fox RF (October 2006). "Kinesin's biased stepping mechanism: amplification of neck linker zippering". Biophys. J. 91 (7): 2416–26. Bibcode 2006BpJ....91.2416M. doi:10.1529/biophysj.106.087049. PMC 1562392. PMID 16844749. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1562392.
- ^ Gross SP, Vershinin M, Shubeita GT (June 2007). "Cargo transport: two motors are sometimes better than one". Current Biology : CB 17 (12): R478–86. doi:10.1016/j.cub.2007.04.025. PMID 17580082.
- ^ Hancock WO (August 2008). "Intracellular transport: kinesins working together". Current Biology : CB 18 (16): R715–7. doi:10.1016/j.cub.2008.07.068. PMID 18727910.
- ^ Kunwar A, Vershinin M, Xu J, Gross SP (August 2008). "Stepping, strain gating, and an unexpected force-velocity curve for multiple-motor-based transport". Current Biology : CB 18 (16): 1173–83. doi:10.1016/j.cub.2008.07.027. PMID 18701289.
- ^ Klumpp S, Lipowsky R (November 2005). "Cooperative cargo transport by several molecular motors". Proceedings of the National Academy of Sciences of the United States of America 102 (48): 17284–9. arXiv:q-bio/0512011. Bibcode 2005PNAS..10217284K. doi:10.1073/pnas.0507363102. PMC 1283533. PMID 16287974. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1283533.
- ^ Rice S, Lin AW, Safer D, Hart CL, Naber N, Carragher BO, Cain SM, Pechatnikova E, Wilson-Kubalek EM, Whittaker M, Pate E, Cooke R, Taylor EW, Milligan RA, Vale RD (December 1999). "A structural change in the kinesin motor protein that drives motility". Nature 402 (6763): 778–84. Bibcode 1999Natur.402..778R. doi:10.1038/45483. PMID 10617199.
- ^ Ambrose JC, Li W, Marcus A, Ma H, Cyr R (April 2005). "A minus-end-directed kinesin with plus-end tracking protein activity is involved in spindle morphogenesis". Mol. Biol. Cell 16 (4): 1584–92. doi:10.1091/mbc.E04-10-0935. PMC 1073643. PMID 15659646. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1073643.
- ^ Yildiz A, Tomishige M, Vale RD, Selvin PR (2004). "Kinesin Walks Hand-Over-Hand". Science 303 (5658): 676–8. Bibcode 2004Sci...303..676Y. doi:10.1126/science.1093753. PMID 14684828.
- ^ Asbury CL (2005). "Kinesin: world’s tiniest biped". Current Opinion in Cell Biology 17 (1): 89–97. doi:10.1016/j.ceb.2004.12.002. PMID 15661524.
- ^ Sindelar CV, Downing KH (February 2010). "An atomic-level mechanism for activation of the kinesin molecular motors". Proc Natl Acad Sci U S A 107 (9): 4111–6. doi:10.1073/pnas.0911208107. PMC 2840164. PMID 20160108. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2840164. Lay summary – Phsyorg.com.
- ^ Atzberger PJ, Peskin CS (January 2006). "A Brownian Dynamics model of kinesin in three dimensions incorporating the force-extension profile of the coiled-coil cargo tether". Bull. Math. Biol. 68 (1): 131–60. doi:10.1007/s11538-005-9003-6. PMID 16794924.
- ^ Peskin CS, Oster G (April 1995). "Coordinated hydrolysis explains the mechanical behavior of kinesin". Biophys. J. 68 (4 Suppl): 202S–210S; discussion 210S–211S. PMC 1281917. PMID 7787069. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1281917.
- ^ Mogilner A, Fisher AJ, Baskin RJ (July 2001). "Structural changes in the neck linker of kinesin explain the load dependence of the motor's mechanical cycle". J. Theor. Biol. 211 (2): 143–57. doi:10.1006/jtbi.2001.2336. PMID 11419956.
- ^ Goshima G, Vale RD (August 2005). "Cell cycle-dependent dynamics and regulation of mitotic kinesins in Drosophila S2 cells". Mol. Biol. Cell 16 (8): 3896–907. doi:10.1091/mbc.E05-02-0118. PMC 1182325. PMID 15958489. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1182325.
Further reading
- Lawrence CJ, Dawe RK, Christie KR, Cleveland DW, Dawson SC, Endow SA, Goldstein LS, Goodson HV, Hirokawa N, Howard J, Malmberg RL, McIntosh JR, Miki H, Mitchison TJ, Okada Y, Reddy AS, Saxton WM, Schliwa M, Scholey JM, Vale RD, Walczak CE, Wordeman L (October 2004). "A standardized kinesin nomenclature". J. Cell Biol. 167 (1): 19–22. doi:10.1083/jcb.200408113. PMC 2041940. PMID 15479732. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2041940.
External links
- Animated model of kinesin walking
- Ron Vale's iBioSeminars on Motor Proteins
- Animation of kinesin movement ASCB image library
- Kinesin and Dynein Microtubule Movement
- The Inner Life of a Cell, 3D animation featuring a Kinesin transporting a vesicle
- The Kinesin Homepage
- MeSH Kinesin
- EC 3.6.4.4
- EC 3.6.4.5
Proteins of the cytoskeleton Human I (MYO1A, MYO1B, MYO1C, MYO1D, MYO1E, MYO1F, MYO1G, MYO1H) · II (MYH1, MYH2, MYH3, MYH4, MYH6, MYH7, MYH7B, MYH8, MYH9, MYH10, MYH11, MYH13, MYH14, MYH15, MYH16) · III (MYO3A, MYO3B) · V (MYO5A, MYO5B, MYO5C) · VI (MYO6) · VII (MYO7A, MYO7B) · IX (MYO9A, MYO9B) · X (MYO10) · XV (MYO15A) · XVIII (MYO18A, MYO18B) · LC (MYL1, MYL2, MYL3, MYL4, MYL5, MYL6, MYL6B, MYL7, MYL9, MYLIP, MYLK, MYLK2, MYLL1)OtherOtherEpithelial keratins
(soft alpha-keratins)Hair keratins
(hard alpha-keratins)Ungrouped alphaNot alphaType 3Type 4Type 5KinesinsOtherOtherNonhuman Hydrolases: acid anhydride hydrolases (EC 3.6) 3.6.1 3.6.2 3.6.3-4: ATPase 3.6.3Cu++ (3.6.3.4)Ca+ (3.6.3.8)Na+/K+ (3.6.3.9)H+/K+ (3.6.3.10)ATP4AOther P-type ATPase3.6.43.6.5: GTPase 3.6.5.1: Heterotrimeric G protein3.6.5.2: Small GTPase > Ras superfamily3.6.5.3: Protein-synthesizing GTPase3.6.5.5-6: Polymerization motorsCategories:- Motor proteins
Wikimedia Foundation. 2010.